Step-by-Step Guide to ISO 13485 Certification in the UK
Summary:
- UK ISO 13485 certification is essential for organisations in the medical device sector, guiding them from design to production, and installation to servicing to meet customer and regulatory demands. It assures adherence to high safety and performance standards.
- Obtaining UK Quality Management Certification involves studying requirements, conducting a gap analysis, and developing an implementation plan, along with providing comprehensive training and conducting internal audits.
- Maintaining UK ISO 13485 certification through surveillance audits and notifying of business changes is essential, signifying a commitment to quality in the UK medical device market.

Introduction
In the dynamic world of medical devices, adhering to stringent quality standards is paramount. Today we provide step by step guide to ISO 13485 certification in the UK services through this post.
ISO 13485 stands out as a beacon of excellence, setting forth requirements for a robust quality management system (QMS) specifically tailored to medical devices.
Achieving ISO 13485 certification signifies more than just a milestone; it reflects a company’s steadfast commitment to quality, assuring customers and regulators of adherence to the highest standards of safety and performance for UK ISO 13485.
This guide simplifies the certification process for UK Quality Management Certification using ISO 13485 standards, facilitating a smoother path to certification.
Why ISO 13485 Certification in the UK is Important?
ISO 13485 is crucial for UK organizations handling medical devices, guiding them in design, production, installation, and servicing. It aids both internal and external parties, like certification bodies, in auditing.
ISO 13485 outlines a QMS that showcases an organization’s capability to consistently meet customer and regulatory demands in providing medical devices and services.

Achieving UK ISO 13485: A Step-by-Step Journey to Excellence
Embarking on UK ISO 13485 certification is like setting sail toward excellence in medical devices. Here’s a step-by-step guide to navigate this journey smoothly:
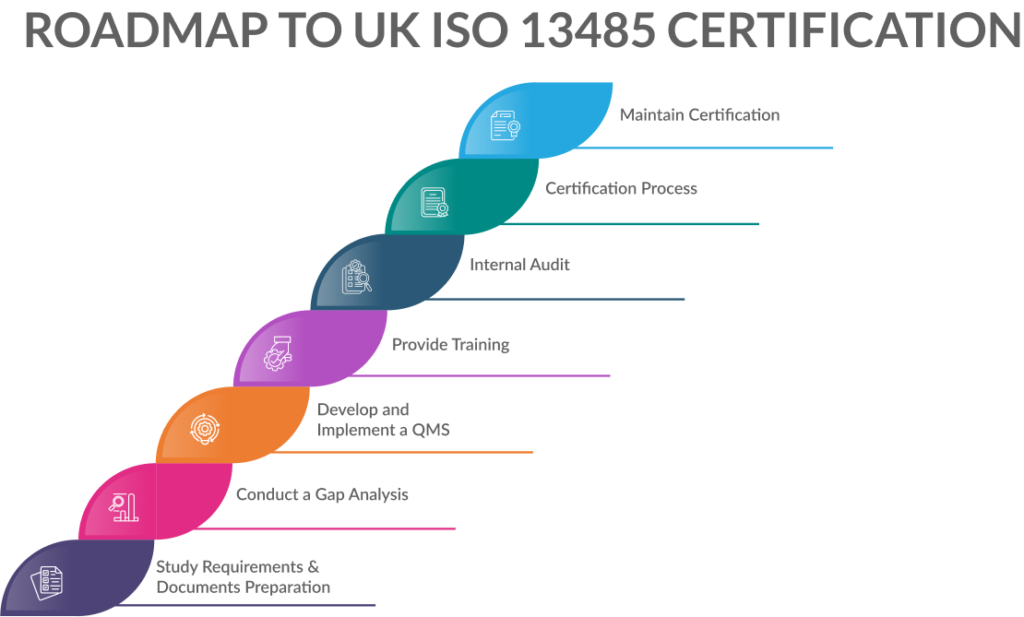
Step 1: Study Requirements & Documents Preparation
To begin, familiarize yourself with the specific QMS requirements applicable to your product category in the UK market. Obtain and study the 2016 version of the ISO 13485 standard, as it contains important changes.
Ensure compliance with ISO 13485 and regulatory requirements in your documentation.
Next, document your QMS processes. Create and maintain an effective quality manual, medical device file, and control of documents and records.
Manage QMS processes according to ISO 13485 and applicable regulations, evaluating changes for their impact.
Step 2: Conduct a Gap Analysis
Perform a gap analysis to align your current processes with ISO 13485.
During the gap analysis, you will:
- Compare your QMS with ISO 13485 requirements.
- Document areas of compliance and non-compliance.
- Use the results to plan your implementation strategy.
After completing the gap analysis, you’ll have a report that highlights:
- Areas where your company meets ISO 13485 requirements.
- Areas where your company falls short and needs improvement.
- Recommendations for your implementation plan.
Work closely with a QMS consultant to conduct a thorough gap analysis, ensuring your QMS is aligned with UK regulations and ISO 13485 standards.
Step 3: Develop and Implement a QMS
Embark on the development and implementation of QMS aligned with ISO 13485 by focusing on key areas:
- Define and document all company processes, ensuring consistency and compliance.
- Create an implementation plan with clear objectives and realistic timelines.
- Design your quality manual and policy, updating processes as necessary.
- Define the scope of your implementation to ensure it meets ISO 13485 requirements.
- Consider responsibilities, documentation, approvals, training, resources, costs, benefits, and business case for certification.
Commit to a QMS that not only meets regulatory standards but also enhances your organization’s overall quality and efficiency.
Step 4: Provide Comprehensive Training
Equip all employees with a thorough understanding of the QMS and their specific roles within it. Conduct training sessions covering the basics of ISO 13485 and any relevant process changes.
Ensure employees are informed well in advance to minimize disruptions. Emphasize the benefits of ISO 13485 implementation to gain their buy-in.
Allow sufficient time for employees to complete training and address any queries before proceeding with implementation.
Step 5: Internal Audit
Before seeking certification, conduct thorough internal audits and management reviews to evaluate the effectiveness of your QMS and compliance with ISO 13485 requirements.
- Develop an internal ISO 13485 audit checklist to assess the functionality of your QMS processes.
- Document your findings to identify areas for improvement and ensure compliance.
- Address any non-conformances through corrective action plans.
- Evaluate QMS process data and resource allocation during a management review. Ensure processes have the necessary resources for effectiveness and continual improvement.
By conducting these audits and reviews, you can identify and rectify any shortcomings in your QMS before undergoing third-party certification audits.
Step 6: Certification Process
Choose a certification body with relevant experience and accreditation. Submit an application with your organization’s details and implementation process.
The process includes a stage one assessment to confirm readiness and a stage two audit for full compliance. Correct any non-conformities. Upon successful completion, the certification body will issue ISO 13485 certification valid for three years.
Step 7: Maintain Certification
To maintain your ISO 13485 certification, undergo an annual surveillance audit to verify compliance and improvements. Notify your certification body of any changes to your business, such as staff size or locations.
These changes may require modifications to your QMS or certification scope. The three-year certification cycle includes ongoing surveillance audits and concludes with a recertification audit to renew the certification.
The journey to achieve UK ISO 13485 certification requires meticulous planning, implementation, and continuous improvement to ensure adherence to regulatory standards and excellence in medical devices.
Conclusion
In conclusion, achieving ISO 13485 certification is a significant milestone for organizations in the medical device sector, representing a UK Quality Management Certification for medical devices.
By following this step-by-step guide, newcomers can better understand and prepare for the certification process, ultimately improving their organisation’s quality management system and ensuring the delivery of safe and effective medical devices.
It demonstrates a commitment to excellence and compliance with stringent quality standards. This certification not only enhances the organization’s reputation but also assures customers of the highest standards of safety and performance in UK ISO 13485 Services.
References
- ISO. ISO 13485 Medical Devices [Internet]. ISO. 2016. Available from: https://www.iso.org/iso-13485-medical-devices.html
- Medical Devices -Quality Management Systems – Requirements for Regulatory Purposes [Internet]. Available from: https://dms.csoftintl.com/wp-content/uploads/2018/01/ISO-13485-2016-EN.pdf
- ISO 13485 Accreditation – Medical Device Quality Management [Internet]. UQAS | Universal Quality Accreditation Services. [cited 2024 May 15]. Available from: https://www.uqas.org/iso-13485-accreditation/
- TheKnowledgeAcademy. Major Benefits of ISO 13485 with Examples [Internet]. www.theknowledgeacademy.com. [cited 2024 May 15]. Available from: https://www.theknowledgeacademy.com/blog/benefits-of-iso-13485/
- Top 5 Benefits of ISO 13485 [Internet]. Intellect. Available from: https://intellect.com/blog/top-5-benefits-of-iso-13485/
- Full Guide to ISO 13485 – Medical Devices | NQA [Internet]. www.nqa.com. [cited 2024 May 15]. Available from: https://www.nqa.com/en-gb/resources/blog/february-2017/a-guide-to-iso-13485#how-to-implement
- ISO 13485 UKAS MEDICAL DEVICES -QUALITY MANAGEMENT SYSTEMS -REQUIREMENTS FOR REGULATORY PURPOSES Certification Process [Internet]. [cited 2024 May 15]. Available from: https://www.sgs.co.uk/-/media/local/uk/documents/technical-documents/sgs-standards/iso-13485-certification-process.pdf
- Imam W. ISO 13485 Implementation – the Complete guide, Including a Checklist [Internet]. advisera.com. [cited 2024 May 15]. Available from: https://advisera.com/13485academy/knowledgebase/checklist-of-iso-13485-implementation-and-certification-steps/
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree regulatory solutions to Medical Devices and In-Vitro Diagnostics.
CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




