Services CliniExperts Offer
CliniExperts is an industry leader in the world of medical devices and IVD regulatory consulting. We are equipped with the knowledge of international regulations and product development solutions and provide services in the U.S., UK, and European markets. Here is a quick overview of our services:

United Kingdom
Market

European Union
Market

USA Market
Regulatory Bodies
Navigate the complex landscape of regulatory bodies in the EU and UK markets with confidence, as we guide you towards successful approvals and compliance.
MHRA
The Medicines and Healthcare products Regulatory Agency is an agency of the Department of Health and Social Care in the United Kingdom. It is the regulatory body responsible for ensuring that medicine, medical devices and blood components for transfusion meet all safety and efficacy standards for optimal public health.
EDQM
The European Directorate for the Quality of Medicines & HealthCare is a directorate of the Council of Europe that was founded with the vision of creating a mainstream European Pharmacopoeia. It is responsible for establishing ethical documentary standards and quality control for medicine, medical devices, cosmetics, and other health products to promote and protect human and animal health.
EMA
The European Medicines Agency is an agency of the European Union in charge of the evaluation and regulation of pharmaceutical products in the EU for human and animal health.
NRAs
NRAs are national regulatory agencies responsible for ensuring that products (medical devices and IVDs) released for public distribution are evaluated properly and meet international standards of quality, safety, and efficacy
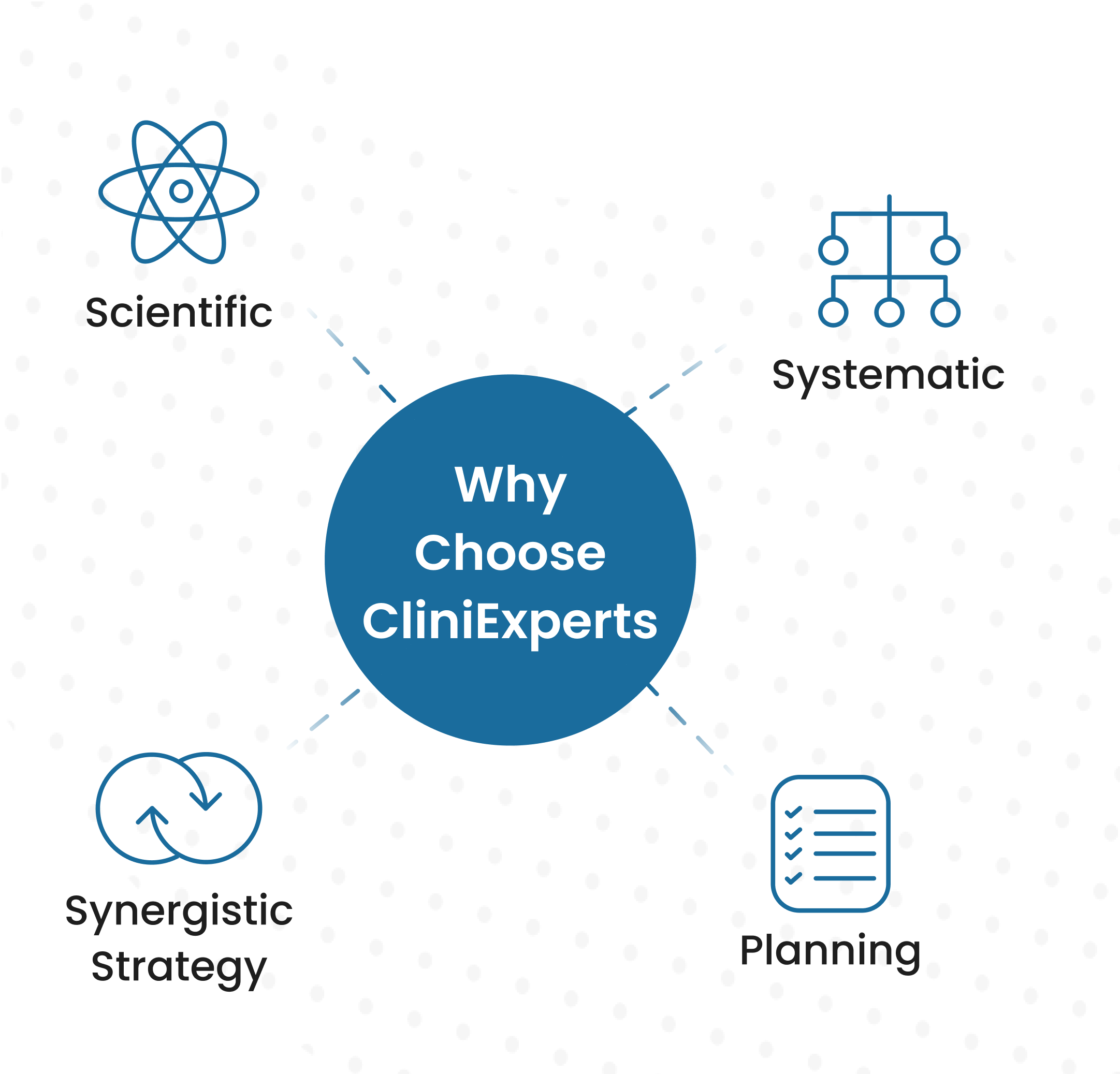
About CliniExperts
Established in 2009 by Dr. Ashwini Kumar, CliniExperts is a leading provider of Regulatory and Clinical Research Services in the EU, U.S., and UK. With ISO 9001 and ISO 27001 certifications, we prioritize strict adherence to Quality Management Systems and ensure top-notch standards in our services.
Our team of passionate and skilled professionals collaborates with global companies known for their high-quality products, services, and customer care. We specialize in assisting companies across various sectors, including Medical Devices, In-Vitro Diagnostics, and more.
At CliniExperts, we live by our belief in unwavering quality and pursuit of excellence. Our zero-tolerance policy for defects creates a flawless regulatory ecosystem, enhancing the ease of doing business for our clients.
Choose CliniExperts as your trusted partner for regulatory compliance and streamlined approvals in the EU, U.S., and UK.


Certifications
ISO 9001:2015 and 27001:2013

Commitment to
Compliance

Team of Expert
Professionals
Our Happy Clients
Our Network Spans the Globe. Proudly serving for over 22 esteemed clients worldwide.
We collaborate with top-notch international companies that have a global reputation for their exceptional products, services, post-sales support, and customer satisfaction. They are dedicated to becoming an integral part of the Indian Healthcare Sector.




























Testimonials

Cliniexperts are highly experienced in Indian regulatory, and the consultation provided is very quick and accurate. They are adaptable to the client needs and timely at the deliverables. We are very much satisfied with the services provided by Cliniexperts and would like to continue collaborating with them for our future research.
Ramya
Remedy

Working with CliniExperts has been an exceptional experience for our company KYOWA. Dr. Kumar and his knowledgeable team have a deep understanding of regulatory guidelines in India, coupled with their professionalism and expertise, which have greatly contributed to the success of KYOWA projects. The team at CliniExperts is dedicated, and responsive, and goes above and beyond to ensure that all aspects of regulatory compliance are met seamlessly. Their commitment to delivering top-notch services, backed by their years of industry experience, has been invaluable to our company KYOWA. We have completed numerous projects with CliniExperts, and their consistent performance and support have solidified our partnership. We highly recommend CliniExperts for its comprehensive regulatory solutions and unwavering commitment to excellence.
Read moreJaime C.
Division: Regulatory Affairs & Quality Assurance - Kyowa Hakko Bio Singapore Pte Ltd
Blog
21 CFR 801 vs. 21 CFR 803: Understanding Labelling and Reporting Regulations
Summary Short Description The 21 CFR 801 and 21 CFR 803 provide medical device regulations and cover labelling and Medical Device Reporting...
The Role of US FDA 510(k) Consultants in Medical Device and IVD Approvals
Summary Short Description Navigating the U.S. Food and Drug Administration’s (FDA) 510(k) premarket notification process is a critical step for medical device...
Understanding the Premarket Approval (PMA) Process: Key Steps for FDA Approval
Introduction Premarket approval (PMA) is a procedure used by the U.S. Food and Drug Administration (FDA) to assess the efficacy and safety...

HAVE A QUERY?
REACH US!
ENQUIRE NOW
Rewards and Recognition








