A Comprehensive Guide To CE Marking Process
Summary:
The CE mark, a mandatory symbol found on various products across Europe, signifies compliance with essential safety, health, and environmental requirements set by the European Union (EU). A manufacturer must apply the EU CE marking to a product for it to be sold in Europe.
To smoothly navigate placing your product in the EEA (European Economic Area), understanding the CE marking process is crucial.

What is CE Marking?
CE stands for “Conformité Européenne,” which is the French term for European conformance. Here is complete guide to CE marking process.
All 27 EU members, as well as Iceland, Norway, and Liechtenstein, must have the mark on products sold in their economies. While many products in Turkey too are required to bear the EU CE marking, Switzerland accepts it only for specific products.
CE Marking Requirements in EU
CE marking is only required for products that meet EU criteria and for which CE marking is required by regulatory authorities. Certain products must simultaneously comply with multiple EU regulations. Before applying the CE mark to your product, you must ensure that it satisfies all applicable regulations.

Overview of the Process Of CE Marking
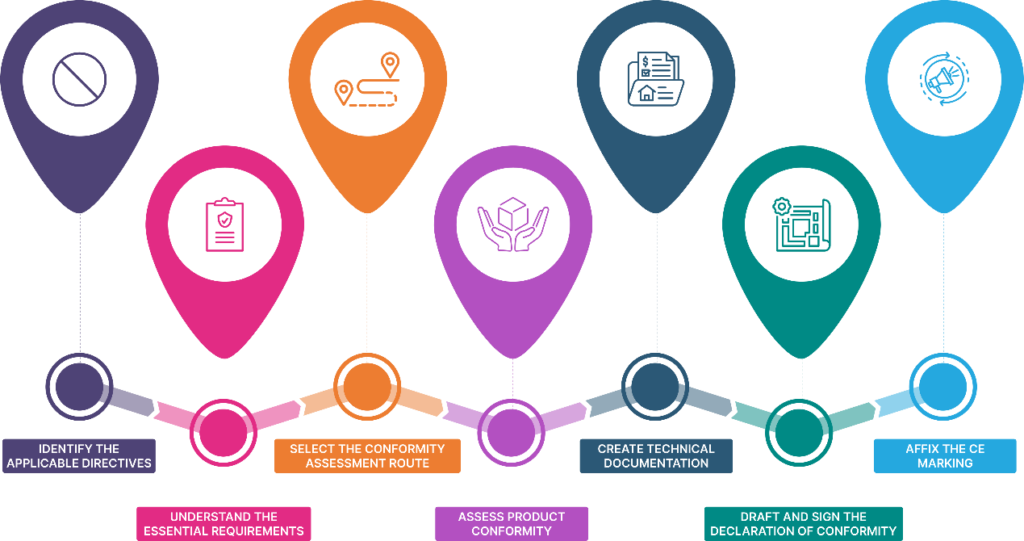
Steps to Obtain CE Mark Certification
Here’s a breakdown of the key steps involved in the process CE marking process:
1. Identifying Applicable Directives
The first step is to determine which EU directives apply to your specific product. These directives outline the essential safety, health, and environmental requirements it must meet. Resources like the EU’s “NANDO” (New Approach Notified and Designated Organisations) database can help you identify relevant directives.
A directive is a piece of EU legislation that mandates member states modify their domestic legal systems to harmonise with EU regulations in this particular area and attain a certain goal.
2. Understanding Essential Requirements for CE Marking
Once you’ve identified the relevant directives, delve into the specific essential requirements outlined for your product category. These detailed requirements form the benchmark for your product’s compliance.
3. Selecting the Conformity Assessment Route
The CE marking process is a self-declaration; however, the level of involvement from a third-party ‘notified body’ varies depending on the directive and product type. Some high-risk products, like medical devices and explosives, might require the mandatory involvement of a notified body for conformity assessment. Explore the various ‘modules’ (assessment procedures) available under each directive to determine the appropriate conformity assessment route for your product.
4. Assessing Product Conformity
This crucial stage involves evaluating your product against the essential requirements. This may involve internal assessments, external testing by accredited laboratories, or a combination of both, depending on the chosen conformity assessment module. There are eight modules for conformance assessment.
- Module A: Internal production control
- Module B: EC-type examination
- Module C: Conformity to type
- Module D: Production quality assurance
- Module E: Product quality assurance
- Module F: Product verification
- Module G: Unit verification
- Module H: Full quality assurance (EN ISO 9001)
The module(s) that are appropriate for a given product category are specified in the applicable directives.
Assessment of conformity typically involves the following:
- Evaluation of the manufacturer’s production quality systems
- Continuous confirmation of product unit compliance
- Reviewing technical documents for product design, manufacturing, and operations.
- Testing of a sample of the items, a typical example of the product, or one or more particular features of each product.
5. Creating Technical Documentation
Compile a comprehensive technical file documenting the processes, design choices, and test results that demonstrate your product’s compliance with the essential requirements. This document serves as evidence of your conformity assessment for future reference.
6. Drafting and Signing the Declaration of Conformity
The Declaration of Conformity (DoC) is a formal document, issued by the manufacturer (or their authorized representative in the EEA), stating that the product meets all relevant directives and essential requirements. It is a crucial element of the CE marking process.
Typically, the Declaration of Conformity contains the following information:
- Your identification details
- What product is it related to
- What regulations are at play
- What standards have been applied
- Where test results are located
- Who is in charge of your organisation
7. Affixing the CE Marking Requirements
Once you’ve completed all the preceding steps and declared conformity with the applicable directives, you can affix the CE marking symbol to your product and its packaging. This signifies your product’s readiness for the European market.
How much do you have to pay for CE Mark Certification?
As a manufacturer, you won’t have to pay any fees if you conduct the conformity assessment yourself. Nevertheless, if the EU specifications applicable to your product require an independent assessment by a notified body, then you must pay the notified body for its services. Costs vary depending on the certification procedure used and the complexity of the product.
Additional Points to Consider for CE Marking Requirements
- Throughout the process, ensure you maintain clear records and documentation related to your conformity assessment.
- Seek guidance from relevant authorities or qualified professionals if needed, especially for complex products or navigating the process for the first time.
- Remember, the CE marking is an ongoing responsibility, and you must ensure your product continues to comply with the relevant directives even after placing it on the market.
Conclusion
The EU CE marking signifies that a product complies with the essential safety, health, and environmental requirements set forth by the European Union (EU). Certain products must be CE-marked before they can be placed on the market in the European Economic Area (EEA). Following these steps will equip you with the essential understanding of the CE marking process, allowing you to navigate the path to bringing your product to the European market with confidence.
References
- CE Marking – EUR-Lex [Internet]. Europa. eu. [cited 2024 Feb 26]. Available from: https://eur-lex.europa.eu/EN/legal-content/glossary/ce-marking.html
- Canada GA. Six Steps to CE Marking [Internet]. GAC. 2019 [cited 2024 Feb 26]. Available from: https://www.tradecommissioner.gc.ca/guides/133383.aspx?lang=eng
- CE marking [Internet]. Your Europe. 2024 [cited 2024 Feb 26]. Available from: https://europa.eu/youreurope/business/product-requirements/labels-markings/ce-marking/index_en.htm
- European standards [Internet]. Europa. eu. [cited 2024 Feb 26]. Available from: https://osha.europa.eu/en/european-standards
- L_2014096EN.01000101.xml [Internet]. Europa. eu. [cited 2024 Feb 26]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX%3A32014L0028
- Technical documentation and EU declaration of conformity [Internet]. Your Europe. 2022 [cited 2024 Feb 26]. Available from: https://europa.eu/youreurope/business/product-requirements/compliance/technical-documentation-conformity/index_en.htm
- CE marking [Internet]. Internal Market, Industry, Entrepreneurship and SMEs. [cited 2024 Feb 26]. Available from: https://single-market-economy.ec.europa.eu/single-market/ce-marking_en
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree regulatory solutions to Medical Devices and In-Vitro Diagnostics.
International CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




