The Benefits of Achieving ISO 13485 Certification for Your Medical Device Business in the UK
Summary:
- ISO 13485 certification is essential for UK medical device businesses, ensuring compliance with international standards and UK quality management certification
- It covers the entire product lifecycle, including design, production, and distribution, and applies to both internal and external stakeholders.
- Achieving UK ISO 13485 services can enhance safety, improve efficiency, and boost credibility in the medical device industry.
- This certification is a strategic investment for businesses aiming to excel and meet regulatory requirements.

Introduction
ISO 13485 certification is a crucial step for any medical device business in the UK. It indicates compliance with international standards for quality management systems (QMS) tailored to the medical device sector, ensuring consistent adherence to customer and regulatory demands.
This certification offers many benefits, including meeting legal requirements, ensuring safe and effective products, managing risks, improving processes, and gaining a competitive market edge.
Let’s explore the transformative benefits of ISO 13485 certification with us, your premier partner in UK Quality Management Certification and UK ISO 13485 Services.
Scope of ISO 13485
ISO 13485 has a far-reaching scope, spanning the complete life cycle of medical devices. It addresses the journey from conceptualization to production, post-production, decommissioning, and disposal of a medical device.
The standard also includes activities like storage, distribution, installation, and servicing, along with the provision of related services.
Moreover, the standard applies to both internal and external stakeholders, including certification bodies and supply chain entities, ensuring comprehensive quality management across the entire medical device sector.
Benefits of ISO 13485 Certification
ISO 13485 certification offers a myriad of benefits that can significantly enhance operational efficiency, ensure product safety, and improve overall quality management. These benefits can elevate your medical device business in the UK. Let’s explore the key advantages:
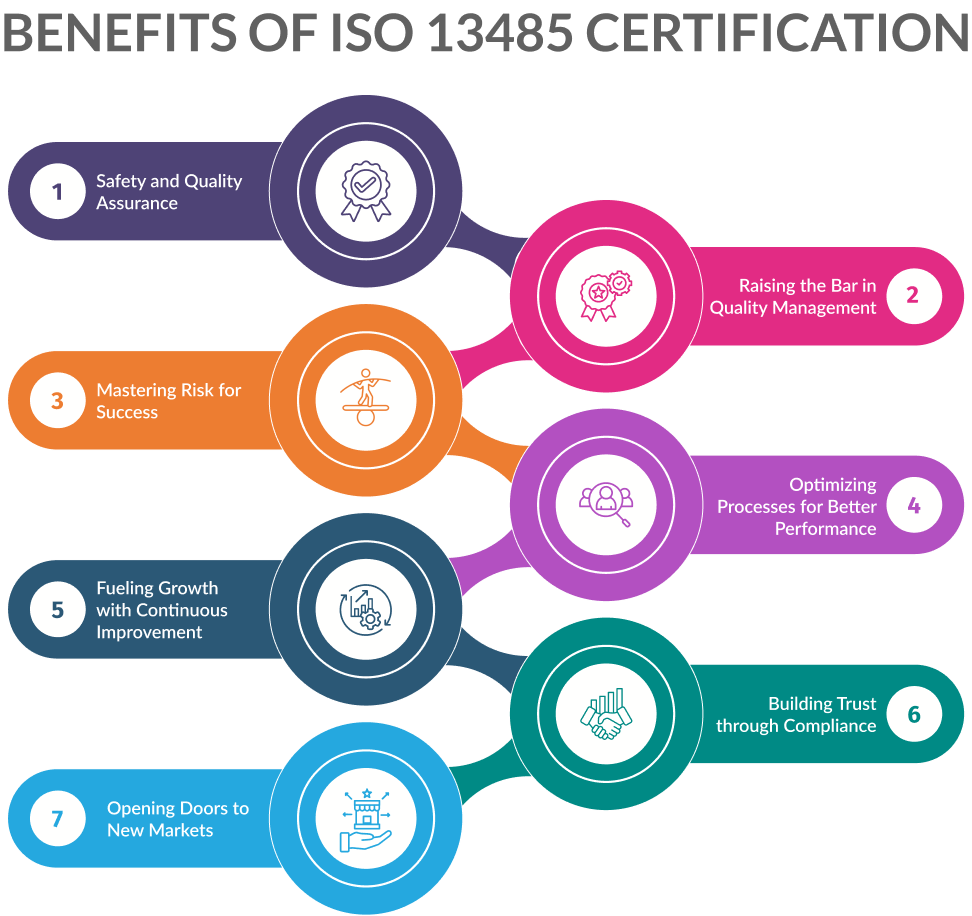
Safety and Quality Assurance
ISO 13485 certification emphasizes the importance of safety and quality in the medical devices industry. It sets stringent regulatory requirements for every stage of a product’s life cycle, including service and delivery.
Certification demonstrates a commitment to meeting these requirements and ensuring best practices.3
Ensuring QMS Practices
ISO 13485 implementation ensures that organizations establish and maintain robust QMS practices. This includes the development of stringent processes for every stage of the medical device lifecycle, from design and production to distribution and servicing.
By adhering to these practices, companies can consistently deliver high-quality products to the market, ensuring their safety and effectiveness.
Moreover, implementing ISO 13485 helps streamline operations and enhances overall quality management. By optimizing processes and workflows, organizations can improve the quality and consistency of their products.
This not only benefits the company internally but also enhances its reputation and credibility in the eyes of customers and stakeholders.
Effective Risk Management
ISO 13485 places a strong emphasis on effective risk management within the medical device industry. Adhering to the standard’s guidelines enables organizations to identify, assess, and mitigate risks related to their products and processes.
This approach minimizes potential hazards and enhances overall safety. ISO 13485 references ISO 14971 to provide further clarification on the requirements and implementation of risk management activities for medical devices.
Credibility and Compliance
ISO 13485 certification demonstrates your commitment to meeting customer expectations, enhancing credibility. This certification enhances your reputation, demonstrating to customers, suppliers, and stakeholders that your products meet international quality standards.
Process Optimization
ISO 13485 drives continuous improvement by encouraging organizations to refine processes and efficiencies. This commitment to best practices leads to streamlined operations, reduced waste, and enhanced efficiency, resulting in cost savings and heightened product quality.
By adhering to ISO 13485 standards, organizations ensure smooth operations through efficient processes, reduced errors, and optimized resource allocation, ultimately improving overall productivity and quality management.
Continuous Enhancement
ISO quality management systems prioritize continual improvement, fostering a culture of innovation within organizations. Adopting a QMS instigates a cultural shift, encouraging management and staff to seek out and implement improvements.
Systematic processes not only mitigate issues and streamline operations but also reduce workloads, promoting high performance, strategic leadership, and employee engagement. These efforts culminate in the delivery of superior products and services, enhancing overall organizational effectiveness.
Market Expansion Potential
ISO 13485 Certification is your passport to new markets, showcasing your steadfast commitment to quality and adherence to international standards.
This certification isn’t just a label; it’s a strategic advantage that propels your organization ahead in the competitive medical device industry. By enhancing your brand reputation and credibility, ISO 13485 opens doors to collaborations and partnerships, expanding your market share and driving overall business growth.
It’s not just about meeting standards; it’s about setting new benchmarks and leading the way in quality management.
ISO 13485 Certified: The Path to Excellence in Medical Devices
Certification to ISO 13485:2003 signifies that an organization has implemented a quality management system (QMS) that meets the requirements of the standard. While certification is not mandatory, it can be beneficial for demonstrating compliance to stakeholders and regulatory bodies.
Organizations certified to ISO 13485:2003 are provided with a three-year transition period to migrate to the new edition of the standard, ISO 13485:2016. This transition period allows organizations to update their QMS to align with the revised requirements.
After the transition period, organizations seeking third-party validation will need to obtain certification to ISO 13485:2016. This certification process involves an independent audit of the organization’s compliance with the new standard.
Elevate Your Standards, Expand Your Horizons!
Achieving ISO 13485 certification for your medical device business in the UK not only demonstrates compliance with international standards but also opens doors to new opportunities.
By adhering to designated standards like ISO 13485 and ISO 14971, you ensure quality management and risk management in your operations, aligning with UK MDR 2002 regulations.
While the use of these standards is optional, most manufacturers opt for them, recognizing the competitive edge and credibility they provide in the market.11
Conclusion
ISO 13485 certification for your medical device business in the UK offers a wide range of benefits, including enhanced credibility, improved operational efficiency, increased customer satisfaction, and access to global markets.
It is a strategic investment that can help your business thrive in an increasingly competitive industry. Achieving UK Quality Management Certification and utilizing UK ISO 13485 Services can pave the way for these advantages, positioning your business for success in the dynamic medical device sector.
References
- International Organization for Standardization. ISO 13485:2016 [Internet]. ISO. 2018. Available from: https://www.iso.org/standard/59752.html
- ISO 13485 ISO 13485 Quality Management for Medical Devices [Internet]. Available from: https://www.iso.org/files/live/sites/isoorg/files/store/en/PUB100377.pdf
- ISO. ISO 13485 Medical Devices [Internet]. ISO. 2016. Available from: https://www.iso.org/iso-13485-medical-devices.html
- ISO 13485 Accreditation – Medical Device Quality Management [Internet]. UQAS | Universal Quality Accreditation Services. Available from: https://www.uqas.org/iso-13485-accreditation/
- Benefits of Using ISO 13485 Quality Management System [Internet]. lfhregulatory.co.uk. 2023 [cited 2024 May 16]. Available from: https://lfhregulatory.co.uk/benefits-of-using-iso-13485/
- Iso.org. 2022 [cited 2022 May 14]. Available from: https://www.iso.org/obp/ui/#iso:std:iso:14971:ed-3:v1:en
- International Organization for Standardization. Certification [Internet]. ISO. 2015. Available from: https://www.iso.org/certification.html
- Medical Devices -Quality Management Systems – Requirements for Regulatory Purposes [Internet]. Available from: https://dms.csoftintl.com/wp-content/uploads/2018/01/ISO-13485-2016-EN.pdf\
- Top 5 Benefits of ISO 13485 [Internet]. Intellect. Available from: https://intellect.com/blog/top-5-benefits-of-iso-13485/
- TheKnowledgeAcademy. Major Benefits of ISO 13485 with Examples [Internet]. www.theknowledgeacademy.com. Available from: https://www.theknowledgeacademy.com/blog/benefits-of-iso-13485/
- GOV.UK. Medical devices: Conformity Assessment and the UKCA Mark [Internet]. GOV.UK. 2020. Available from: https://www.gov.uk/guidance/medical-devices-conformity-assessment-and-the-ukca-mark
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree Global Regulatory Solutions related to Pharma, Medical Devices and In-Vitro Diagnostics.
CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




