Medical devices: conformity assessment and the UKCA mark
Summary:
- Medicines and Healthcare products Regulatory Agency (MHRA) is the regulatory body governing the UKCA Marking in the Great Britain.
- MHRA provides guidance on how the medical devices and in-vitro diagnostic kits are regulated in the UK.
- MHRA has declared a 12-month extension for implementing the Medical Device Regulations (UKCA Mark). The new regulations will be enforced from July 2024.

After separating itself from the European Union, Great Britain introduced UKCA Mark mandatory for medical devices to conform to the new regulations. Any manufacturer who wishes to enter into the UK market must register their medical devices with the Medicines & Healthcare Regulatory Agency (MHRA) of UK within a specified timeframe. Medical devices conformity assessment is also necessary for the medical devices.
This article will provide an overview for conformity assessment and UKCA Mark, the overall timeline for UKCA Mark implementation and what these mean for manufacturers.
Background on UKCA Mark
On 31 Dec 2020, the United Kingdom left the European Union single market. As a result, the United Kingdom introduced a new assessment mark called the United Kingdom Conformity Assessment Mark (UKCA). It was effective from 01 January 2021. This UKCA mark will replace the CE mark in England, Wales, and Scotland.
For medical devices and in vitro diagnostic kits the changeover period was ending on 30 June 2023. Due to such a short period, the medical device manufacturers were under pressure to ensure they were aligned with the new UK requirement. Additionally, due to delays in application and simultaneously introducing new medical devices regulatory frameworks, the MHRA has now declared that they will be extending the UKCA mark implementation timeline to next year i.e., July 2024.
What is Medical Devices Conformity Assessment?
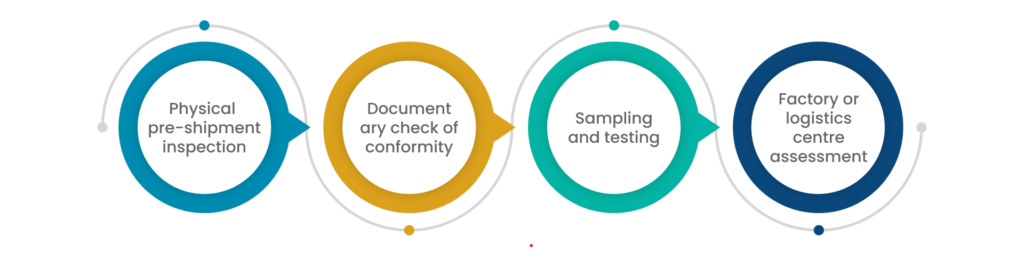
To introduce a medical device on the Great Britain market (England, Wales and Scotland), manufacturers must demonstrate that their medical devices conform to the regulatory requirement as per the Medical Devices Regulation 2002 by conducting the conformity assessment.
There are approved regulatory bodies that conduct conformity assessments of certain medical devices. They ensure that the devices comply with the mandatory requirements of the UK Medical Devices Rules. Suppose the medical devices fulfil the application needs, the higher authorities/approved bodies issue a Certificate of Conformity to the manufacturer that allows them to sell/market their medical devices in Great Britain.
What is United Kingdom Conformity Assessment Mark?
Following its exit from European Union single Market, in December 2022 the Great Britain replaced the CE (Conformité Européenne) mark with UKCA mark for medical devices that were marketed in these territories.
The Authorities called the UKCA as United Kingdom Conformity Assessment Mark. It was made mandatory for all medical devices which indicated that it complies with the new Great Britain legislation and it operates in similar ways as the CE mark in EU.
The manufacturers or their locally authorised representatives are responsible for obtaining a UKCA mark onto their devices. UKCA mark makes the medical devices authorised.
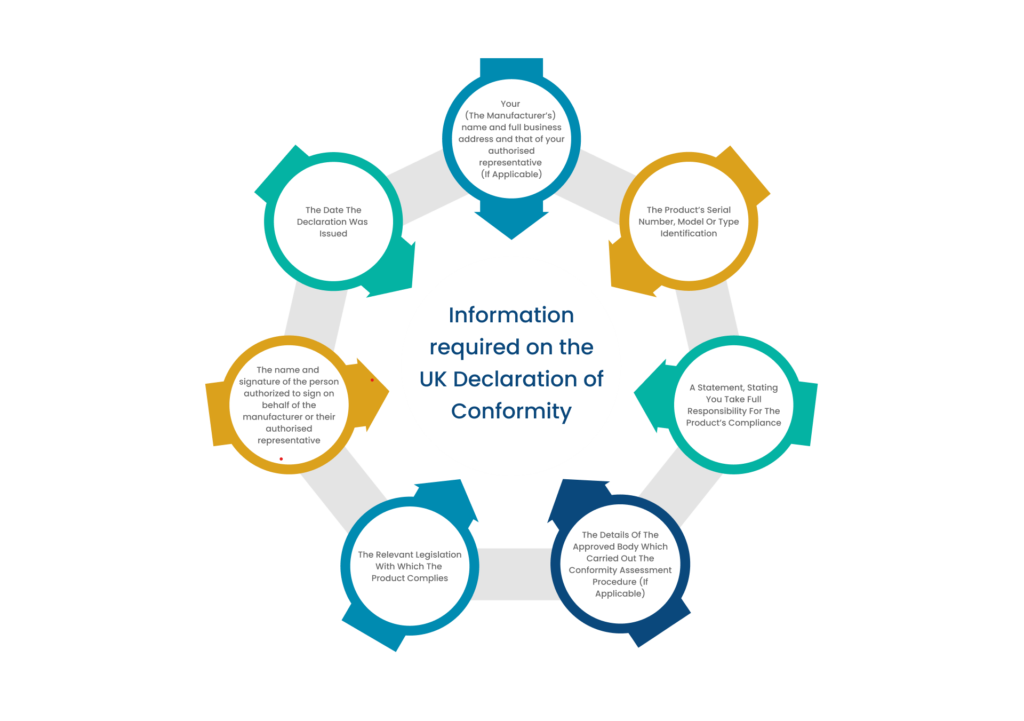
What is the significance of UKCA Mark?
The UKCA Mark is only applicable in Great Britain. The medical devices that will be introduced in these market will therefore, require UKCA Mark. Presence of the UKCA logo on the device shows that:
What is the difference between UKCA and CE Mark?
A medical device may have both UKCA and CE Marking provided it fulfils the relevant necessary requirements of the European Union and UK markets. It is possible to have multiple conformity marks of different regions for the same products sold in various international markets.
What is the timeline for UKCA Mark implementation in the Great Britain?
The MHRA has recently announced an extension of a year on implementing the Medical Device Regulation. This aims to bring the new regulation into force by July 2024. For now, the manufacturers will be able to continue their sale with the CE marked medical devices on Great Britain market after 1 July 2023.
Authorities are planning to introduce the new regulation from July 2024 that will enforce the transitional arrangement for medical devices in the United Kingdom will apply for CE and UKCA marked devices placed Britain market.
| Important dates | Actions |
| January 2021 | UKCA marking enforced |
| January 2021 to June 2024 | Medical devices can be either UKCA or CE marked or can be both. |
| 30 June 2024 | Time limit for medical devices manufacturers to change any existing CE marking with the UKCA marking. |
| 1 July 2024 | The UKCA mark will be mandatory requirement for all the available and newly introduced medical devices on the Great Britain market. CE marking will no longer be recognised. |
(Above timelines maybe subject to change per MHRA updates)
What is the immediate action for the manufacturers?
The immediate action for manufacturers is to be prepared for a hassle-free transition. Stay updated with the new timelines for UKCA marking.
The UKCA Mark majorly affects the manufacturers in Great Britain. Manufacturers should look out for the two important points of impact-
- Product Registration- All the medical devices that are marketed in Great Britain must be registered with the MHRA. Whenever its application, conformity assessment by a UK Approved Body for UKCA needs to be provided during the registration process.
- UK Responsible Person- The manufacturer located outside the UK, will require to appoint a local UK responsible person who will act on their behalf for registering the medical devices with the MHRA.
- CE marking will no longer be recognized in Great Britain until 30 June 2024. Similarly, the EU no longer holds authority as UK Notified Bodies.
- Manufacturers must not panic. This delay in UKCA marking enforcement is a good opportunity to prepare medical devices for regulatory compliance.
- Manufacturers must invest time to get the things correct and compliant with the new regulations, this will ensure that the medical devices can be marketed and used for improving human health.
Reference:
- Chapter 6: Conformity Assessment Background. [Internet]. [cited 2023 Jul 3]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1018211/Chapter_6_Conformity_Assessment_PDF.pdf
- Medical devices: conformity assessment and the UKCA mark – GOV.UK [Internet]. [cited 2023 Jul 3]. Available from: https://www.gov.uk/guidance/medical-devices-conformity-assessment-and-the-ukca-mark
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree regulatory solutions to Medical Devices and In-Vitro Diagnostics.
International CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




