Understanding the Premarket Approval (PMA) Process: Key Steps for FDA Approval

Introduction
Premarket approval (PMA) is a procedure used by the U.S. Food and Drug Administration (FDA) to assess the efficacy and safety of Class III medical devices.
These devices either carry an unreasonable risk of illness or injury, are essential in preserving or extending human life, or significantly contribute to preventing impairment of human health.
Devices classified as class III include those that maintain or support human life, have a significant role in preventing health impairment in humans, or pose an unjustifiable risk of disease or injury.
The FDA has concluded that general and special controls alone are inadequate to ensure the safety and efficacy of Class III devices due to the level of risk associated with these devices.
Therefore, these devices must submit a premarket approval (PMA) application per section 515 of the FD&C Act to receive marketing approval.
Due to the high risk connected with Class III devices, the PMA pathway is the most stringent FDA-approved medical device procedure.
The PMA process includes a thorough scientific and regulatory medical device review. The applicant must present credible scientific proof that the device is safe and effective for the intended use(s).
After that, the FDA will examine the applicant’s data, including manufacturing details, nonclinical test data, and outcomes from clinical trials.
After the FDA approves the device’s PMA application, it can be sold for its intended use or uses. Notably, the FDA’s most stringent and drawn-out regulatory pathway for medical devices is the PMA process, which normally takes several years.
What Must a Premarket approval (PMA) Submission Contain?
Despite its significant importance, the application submission process for premarket approval is surprisingly informal. Although there isn’t a formal PMA form, a premarket approval submission must comply with specific requirements.
- A thoughtfully created cover letter and table of contents
- A concise but comprehensive abstract section that covers the essential details and provides a general overview of the content found in the PMA.
- Outcomes of published non-clinical and clinical laboratory research involving humans. To prevent data contamination, these must be divided into distinct sections.
- Briefly state the illness the device is intended to treat in the indications for use.
- A clear and well-informed description of the device should be provided.
- Explain any potential substitute procedures or applications for the devices.
- All standards met during the design and manufacturing process, including self-imposed and voluntary standards, should be mentioned.
- Conclusions derived from the research and studies on marketing
- Disclosure of any trade or confidential information or materials used throughout; at least once in the text, the author must identify in copy any information that the author knows to be confidential.
- Any relevant changes to the device, marketing strategy, or manufacturing.
Who is eligible for PMA application?
If the company owns the device or has “authorized access” to the data required for the application, anyone working for the company may submit an FDA PMA application.
Information needed to approve a PMA
The following are the topline requirements:
- Cover letter stating the kind of submission one is making.
- Key submission points summarised (10–15 pages, approximately).
- Description of the device; substitutes for it for the ailment or disease it is treating; past marketing.
- Summary of technical data and conclusions.
- Detailed description of the device.
- References to performance/voluntary standards that cover every facet of the effectiveness and safety of the device.
- Technical information.
- Results of non-clinical laboratory research
- Outcomes of clinical trials.
- The rationale for a single investigator.
- Any reports that have been published regarding the device
- Samples are available upon request or at a location for examination.
- Information needed to approve a PMA.
- Any reports that have been published regarding the device.
- Samples upon request or a location for examination.
- Suggested labelling, with any available promotional materials
- Disclosure statement or financial certification
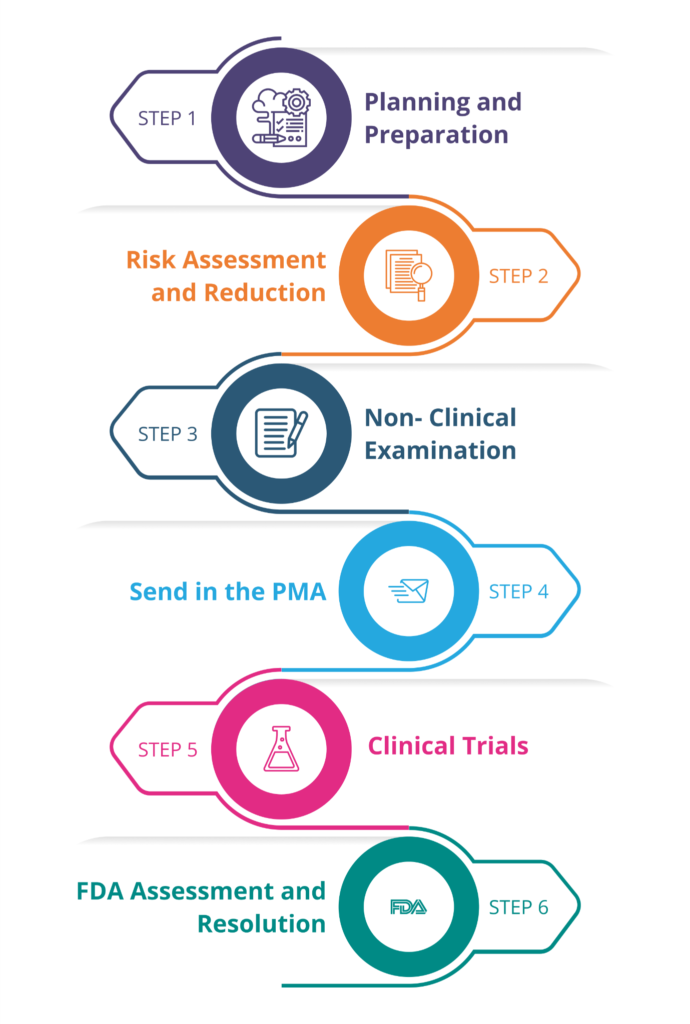
Steps in the PMA Application Process:
Step 1: Planning and Preparation
Recognize the requirements, create data collection and risk analysis plans, and compile the required paperwork.
Step 2: Risk Assessment and Reduction
To ensure patient safety, perform a thorough risk analysis by identifying potential hazards and implementing risk controls.
Step 3: Non-Clinical Examination
Extensive non-clinical testing assesses the device’s performance, materials, design, and possible risks.
Step 4: Send in the Premarket approval (PMA)
Gather and submit all information proving the safety and effectiveness of the device. This ought to comply with the FDA guidelines.
Step 5: Clinical Trials
To gather information about the effectiveness and safety of the device, conduct clinical trials on human subjects.
Step 6: FDA Assessment and Resolution
After reviewing the submission and requesting additional information, the FDA decides whether to approve it.
Review of PMA Submissions
The FDA breaks down the PMA submission review process into four steps, which are as follows:
- FDA employees conduct administrative and restricted scientific reviews to assess completeness (acceptance and filing reviews).
- Thorough examination of the Quality System, science, and regulations by qualified FDA staff (substantive review).
- Evaluation and suggestion from the relevant advisory committee (panel review); and
- Final discussions, record-keeping, and FDA decision notification.
The FDA approves an application for commercial distribution and use in the US market if it satisfies all regulatory requirements and offers solid scientific proof of safety and efficacy.
The FDA might accept the application in rare circumstances but add requirements or conditions to address any issues.
These requirements could be extended labeling information, post-market surveillance studies, or continuous safety monitoring to guarantee continuous device safety.
The FDA issues a “Not Approvable” letter explaining the specific reasons for the decision and highlighting application deficiencies that must be addressed if the application does not meet its safety and effectiveness standards.
Conclusion
The Premarket Approval (PMA) process is a crucial regulatory pathway for ensuring the safety and effectiveness of Class III medical devices in the U.S. market.
Given the high-risk nature of these devices, the PMA process is the most rigorous of the FDA’s regulatory pathways, requiring extensive scientific evidence and detailed documentation.
The PMA process is essential for bringing innovative yet high-risk medical devices to the market, ensuring they are safe and effective for their intended use. This process’s comprehensive and meticulous nature underscores the importance of adhering to regulatory standards to achieve FDA approval.
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree Global Regulatory Solutions related to Pharma, Medical Devices and In-Vitro Diagnostics.
CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




