Streamlining Your CE Marking Process: Tips for Efficiency and Success
Summary:
- Obtaining a CE marking process is crucial for medical device manufacturers seeking to market their products in the EU.
- This conformity marking demonstrates compliance with EU regulations and essential requirements.
- The CE marking process involves several steps including identifying applicable directives, conducting gap analyses, developing a Quality Management System (QMS), compiling technical documentation, and engaging with Notified Bodies.

Introduction
The CE marking is a mandatory conformity marking for products sold in the European Economic Area, demonstrating compliance with applicable EU directives and regulations. It ensures that medical devices meet essential safety, performance, and quality requirements for market entry in the EU.
What Is the CE Marking Process for Medical Devices?
The CE Marking process is a mandatory conformity marking for products sold within the European Economic Area (EEA). It signifies that the product meets the applicable requirements set forth by the European Union (EU) directives and regulations.
Why Is the CE Marking Process Important?
CE Marking process is crucial for medical device manufacturers seeking to market their products in the EU. Products cannot be legally sold or distributed within the EEA without this mark. The marking process ensures that medical devices meet the essential safety, performance, and quality requirements, protecting public health and enabling the free movement of goods within the EU single market.
When Is the CE Marking Process Mandatory?
CE marking process is mandatory solely for products governed by existing EUspecifications that necessitate its application. Certain products are subject to multiple EU requirements concurrently. Ensuring compliance with all pertinent requirements before affixing the CE marking is imperative. Affixing the CE marking to products lacking EU CE Marking specifications or not mandating its application is prohibited.
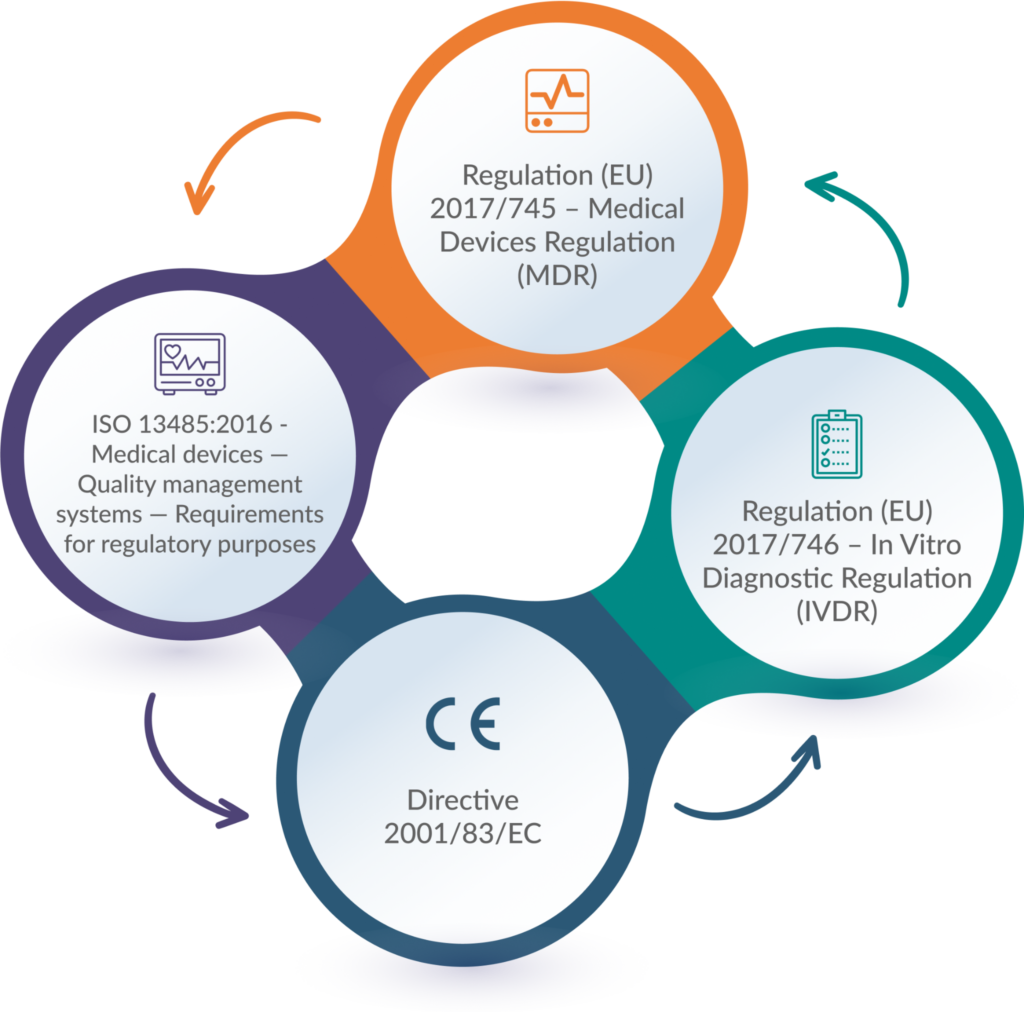
Relevant Standards and Regulations
For medical devices, the CE Marking process demonstrates compliance with the Medical Device Regulation (MDR) or the In Vitro Diagnostic Regulation (IVDR), depending on the product category.
The CE Marking Process for medical devices is governed by the MDR and IVDR, which outline the essential requirements and conformity assessment procedures. They are listed below:
- Regulation (EU) 2017/745 – Medical Devices Regulation (MDR)
The regulation delineates all essential procedures, transition protocols, and clarifications. As a medical device manufacturer, it’s imperative to consistently consult this regulation for precise information.
- Regulation (EU) 2017/746 – In Vitro Diagnostic Regulation (IVDR)
This regulation facilitates the seamless operation of the internal market concerning in vitro diagnostic medical devices, emphasizing a robust level of health protection for patients and users, while also considering the needs of small and medium-sized enterprises.
- Directive 2001/83/EC
Directive 2001/83/EC pertains to the marketing authorization of medicinal products intended for human use.
- ISO 13485:2016 – Medical devices — Quality management systems — Requirements for regulatory purposes
The ISO 13485:2016 standard delineates the criteria for a Quality Management System (QMS) applicable to medical devices.
These regulations are complemented by harmonised standards, which provide technical specifications and guidelines for demonstrating compliance with the CE Marking Requirements in the EU.
How To Obtain CE Marking for Medical Devices?
Obtaining CE Marking for medical devices involves several key steps:
- Identify the applicable directives and regulations based on your product’s classification and intended use.
- Conduct a gap analysis to assess compliance with the essential requirements.
- Develop and implement a robust Quality Management System (QMS) aligned with relevant harmonized standards.
- Compile comprehensive technical documentation, including product specifications, risk assessments, and test reports.
- Engage with a Notified Body for conformity assessment, if required by the applicable directive or regulation.
- Affix the CE marking to the product and issue a Declaration of Conformity.
- Establish post-market surveillance and vigilance processes.
Fees For Obtaining CE Marking Process
Generally, fees for obtaining the CE Marking Process are not required if a manufacturer carries out the conformity process himself. Nevertheless, if they enlist the services of a notified body, or if the EU specifications governing their product necessitate an independent assessment by a notified body, they are obligated to remunerate the notified body for its services. The expenses are contingent upon the certification procedure applicable to their product and factors such as its complexity.
Duration Of Validity for CE Marking
The CE marking does not have a specified period of validity. However, it is essential to maintain the EU Declaration of Conformity (DoC) required for the EU CE marking and ensure it remains current. If any elements of the DoC change, such as legislation, product modifications, or manufacturer or authorized representative contact details, the Declaration must be updated accordingly.
CliniExperts, a leading regulatory consulting firm, offers comprehensive services to guide medical device manufacturers through the CE Marking Process. With a team of experienced regulatory experts and a deep understanding of the CE Marking Requirements in the EU regulatory landscape, CliniExperts can provide valuable assistance at every stage of the process.
By following best practices, leveraging the expertise of regulatory consultants like CliniExperts, and investing in an efficient CE Marking process strategy, medical device manufacturers can pave the way for a successful and timely market entry within the EU CE Marking.
Conclusion
Navigating the complexities of the CE marking process can be daunting, but with the right guidance and expertise, medical device manufacturers can streamline their path to the European market. By following best practices, leveraging the expertise of regulatory consultants like CliniExperts, and investing in an efficient CE Marking process strategy, medical device manufacturers can pave the way for a successful and timely market entry within the EU CE Marking.
References
- CE Marking. [Internet]. [cited 2024 Apr 23] Available from: https://single-market-economy.ec.europa.eu/single-market/ce-marking_en
- CE Marking. [Internet]. [cited 2024 Apr 23] Available from:https://europa.eu/youreurope/business/product-requirements/labels-markings/ce-marking/index_en.htm
- Regulation – 2017/745 – en – medical device regulation – EUR-lex. [Internet]. [cited 2024 Apr 23] Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0745
- Regulation – 2017/746 – en – medical device regulation – EUR-lex. [Internet]. [cited 2024 Apr 23] Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0746
- Lex – 02001L0083-20220101 – en – EUR-Lex. [Internet]. [cited 2024 Apr 23] Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02001L0083-20220101
- Medical devices — Quality management systems. [Internet]. [cited 2024 Apr 23] Available from: https://www.iso.org/obp/ui#iso:std:iso:13485:ed-3:v1:en
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree regulatory solutions to Medical Devices and In-Vitro Diagnostics.
International CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




