EU Quality Management Services, ISO 13485 Auditing Services in EU
Summary:
- ISO 13485 is a globally recognised standard for quality management systems in the medical device industry.
- It specifies requirements for establishing, implementing, and maintaining robust quality management systems to ensure consistent product design, development, production, and servicing.
- Key components of ISO 13485 encompass risk management, quality system documentation, design and development controls, supplier evaluation, and corrective/preventive action processes.
- Achieving ISO 13485 certification involves thorough audits by accredited third-party certification bodies, followed by periodic surveillance audits.
- Benefits for EU medical device manufacturers include regulatory compliance, improved product quality and safety, enhanced customer confidence, streamlined processes, and a competitive edge in the market.

Introduction
With ISO 13485 auditing services in the EU, companies can validate their adherence to these rigorous standards, instilling confidence in stakeholders and enhancing market competitiveness.
A medical device, whether an implant, instrument, machine, or in vitro reagent, is designed for prevention, treatment, or diagnosis of a medical condition.
In the medical devices sector, safety and quality are principal leading to the establishment of strict regulations that devices must adhere to meet EU quality management services.
As regulatory standards tighten, organisations are compelled to showcase rigorous quality management processes.
This has led to the development of the globally acknowledged ISO 13485 standard that outlines precise mandates for establishing a tailored quality management system within the medical devices industry.
What is ISO 13485?
ISO 13485 stands as a worldwide recognized standard for quality management systems (QMS), meticulously crafted for the medical device sector.
It describes the prerequisites for instituting, executing, and sustaining a resilient QMS, guaranteeing the uniformity of medical device design, development, production, installation, and servicing, all while meeting customer and regulatory stipulations.
The primary aim of the ISO 13485 standard is to establish standardized EU quality management services and is based on the framework of the ISO 9001 standard.
ISO 13485 shares similar clauses, with some directly referencing ISO 9001 and serves as the cornerstone for regulatory adherence in both local and international markets making this certification essential for the global exportation of medical devices.
Key Components of ISO 13485 Auditing Services in EU
ISO 13485 encompasses a range of requirements spanning various aspects of medical device manufacturing. Here are some of the key elements of the EU quality management services:
- Risk Management: Manufacturers are required to develop and uphold a strong risk management procedure to detect, assess, and address potential risks linked to their medical devices.
- Documentation of the Quality System: Thorough documentation encompassing quality policies, procedures, and records is indispensable. These documents oversee the entirety of the medical device lifecycle, from design and development to production and post-market surveillance.
- Design and Development Controls: Several controls are necessary during the design and development phases to guarantee alignment with user needs, intended usage specifications, and relevant regulatory standards.
- Supplier Assessment and Management: Manufacturers are obligated to assess and oversee their suppliers to verify that acquired products and services adhere to defined specifications.
- Corrective and Preventive Measures: Procedures must be established to rectify non-compliances, investigate underlying causes, and execute corrective and preventive actions to deter reoccurrence.
| Element | Description |
| Risk Management | Identify, analyse, and mitigate potential risks associated with medical devices |
| Quality System Documentation | Comprehensive documentation governing design, development, production, and post-market surveillance |
| Design and Development Controls | Ensure medical devices meet user needs, intended use requirements, and regulatory requirements |
| Supplier Evaluation and Control | Monitor suppliers to ensure purchased products and services meet specified requirements |
| Corrective and Preventive Action | Address non-conformities, investigate root causes, and implement corrective and preventive actions |
Achieving ISO 13485 Certification:
To achieve ISO 13485 certification, manufacturers must undergo a comprehensive audit process conducted by an accredited third-party certification body. The outlined four steps are:
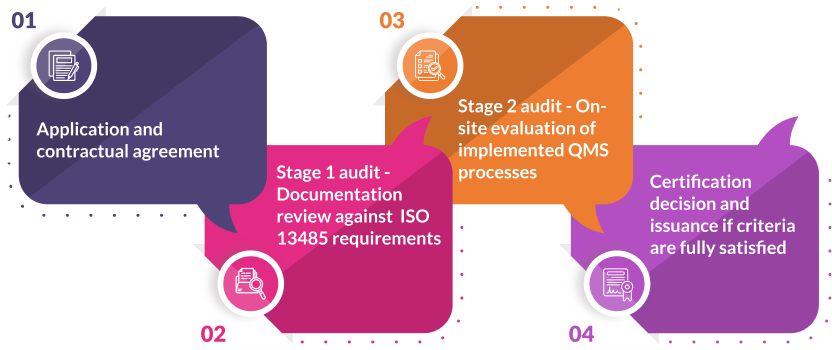
- Application and contract signing for financial terms
- Stage 1 Audit – Documentation review for compliance
- Stage 2 Audit – On-site audit to evaluate QMS application and effectiveness
- Certificate issued if all criteria are met
Once certified, manufacturers must maintain their QMS and undergo periodic surveillance audits to ensure ongoing compliance.
Recertification audits are typically conducted every three years to renew the ISO 13485 certification.
Benefits of ISO 13485 for Medical Device Manufacturers in the EU
Implementing a robust EU quality management services system through ISO 13485 certification is crucial for medical device manufacturers seeking to enter and thrive in the European Union market. Benefits include:
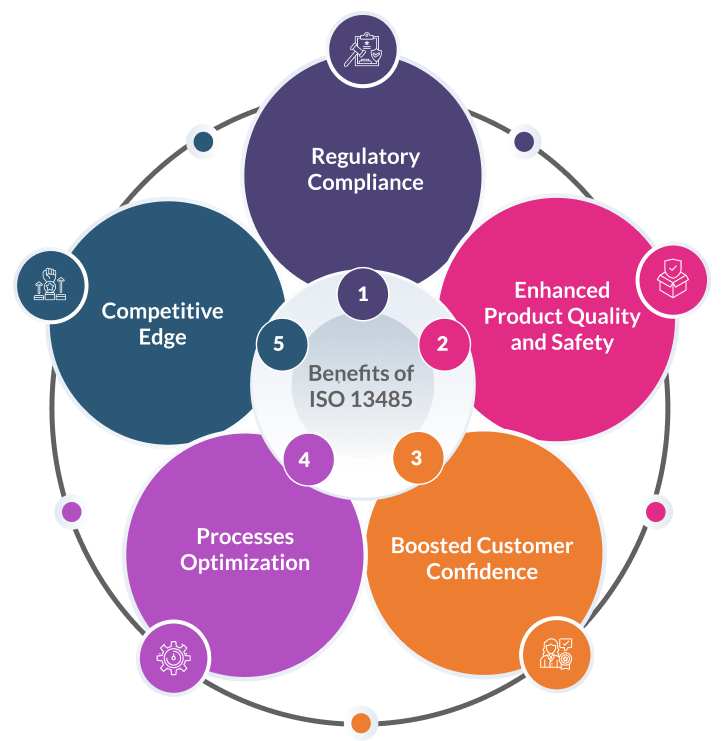
- Regulatory Compliance: Compliance with ISO 13485 signifies adherence to the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), easing market entry and minimising regulatory obstacles.
- Enhanced Product Quality and Safety: By implementing a robust quality management system, ISO 13485 ensures consistent and reliable design, development, and production processes, resulting in medical devices of superior quality and heightened safety.
- Boosted Customer Confidence: ISO 13485 certification serves as a globally recognised mark of excellence, inspiring trust among customers and end-users, and reinforcing confidence in the products offered.
- Process Optimization: The structured approach to quality management advocated by ISO 13485 promotes efficiency and optimisation of processes, reducing inefficiencies and enhancing overall productivity.
- Competitive Edge: Manufacturers holding ISO 13485 certification gain a competitive advantage in the EU market, positioning themselves as reputable and quality-oriented suppliers, thereby increasing their market appeal and opportunities for growth.
As a premier provider of ISO 13485 Auditing Services in EU, we recognize the critical role of thorough documentation in meeting regulatory requirements like the Medical Device Regulation (MDR) in the European Union.
Our specialized team offers customized solutions to navigate the intricate documentation mandates of ISO 13485, ensuring transparency, traceability, and compliance with the demanding standards for product safety and quality management systems stipulated by the MDR.
Conclusion
For medical device manufacturers in the EU, grasping and applying ISO 13485 is essential.
This globally acknowledged standard in quality management systems, facilitated by EU Quality Management Services, guarantees regulatory adherence, heightens product quality, and implants customer trust.
By adhering to ISO 13485, manufacturers can demonstrate their commitment to excellence and gain a competitive advantage in the highly regulated medical device industry within the European Union.
With the support of CliniExperts ISO 13485 Auditing Services in the EU, companies can navigate the complexities of certification and ensure seamless integration of ISO 13485 standards into their operations.
References
- ISO 13485:2016. [Internet]. [cited 2024 May 15] Available from: https://www.iso.org/standard/59752.html
- Medical Devices. [Internet]. [cited 2024 May 15] Available from: https://www.iso.org/iso-13485-medical-devices.html
- ISO 13485:2016. ISO India [Internet]. [cited 2024 May 15] Available from:https://isoindia.org/iso_13485.php
- ISO 13485: A Complete Guide to Quality Management in the Medical Device Industry. [Internet]. [cited 2024 May 15] Available: https://books.google.co.in/books
- Jadhav, Nitin & Shendge, Raosaheb. (2024). ISO 13485:2016 – The Gateway of Global or Regional Harmonization for Medical Device Regulations. INTERNATIONAL JOURNAL OF PHARMACEUTICAL QUALITY ASSURANCE. 15. 502-511. 10.25258/ijpqa.15.1.76. Available from:https://impactfactor.org/PDF/IJPQA/15/IJPQA,Vol15,Issue1,Article76.pdf
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree regulatory solutions to Medical Devices and In-Vitro Diagnostics.
CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




