Compliance Consulting for Medical Device Registration in EU
Summary:
Compliance consulting is crucial in navigating the stringent EU medical device regulations (MDR). Consultants provide vital guidance, ensuring manufacturers meet rigorous standards for market approval, enhance product safety, and maintain regulatory compliance throughout the device lifecycle.

Introduction
Navigating the regulatory landscape of medical device registration in the European Union (EU) can be complex and daunting.
EU Medical Device consulting offers essential guidance to ensure that medical devices being manufactured or sold in the EU meet all regulatory requirements, enabling smooth market entry and ongoing compliance.
This service helps manufacturers adhere to stringent MDR standards through comprehensive product evaluations, development of technical documentation, implementation of risk management strategies, and post-market surveillance.
This equips manufacturers to competently and efficiently navigate the intricate regulatory framework.
Understanding EU Medical Device Regulations
MDR and In Vitro Diagnostic Regulation (IVDR) are the primary regulatory frameworks governing medical devices in the EU.
These regulations replaced the previous Medical Device Directive (MDD) and In Vitro Diagnostic Directive (IVDD) to enhance safety and performance standards.
Key requirements for manufacturers include ensuring compliance with either the MDR or IVDR, implementing a Quality Management System (QMS) aligned with ISO 13485, conducting clinical evaluations, preparing technical documentation, and obtaining CE marking.
Notified Bodies (NBs) play a crucial role in this process by assessing device conformity, ensuring adherence to EU regulations, and certifying devices for market approval.
Why is Compliance Essential for Medical Device Manufacturers?
Compliance, facilitated by EU medical device consulting services, is crucial for medical device manufacturers due to several compelling reasons.
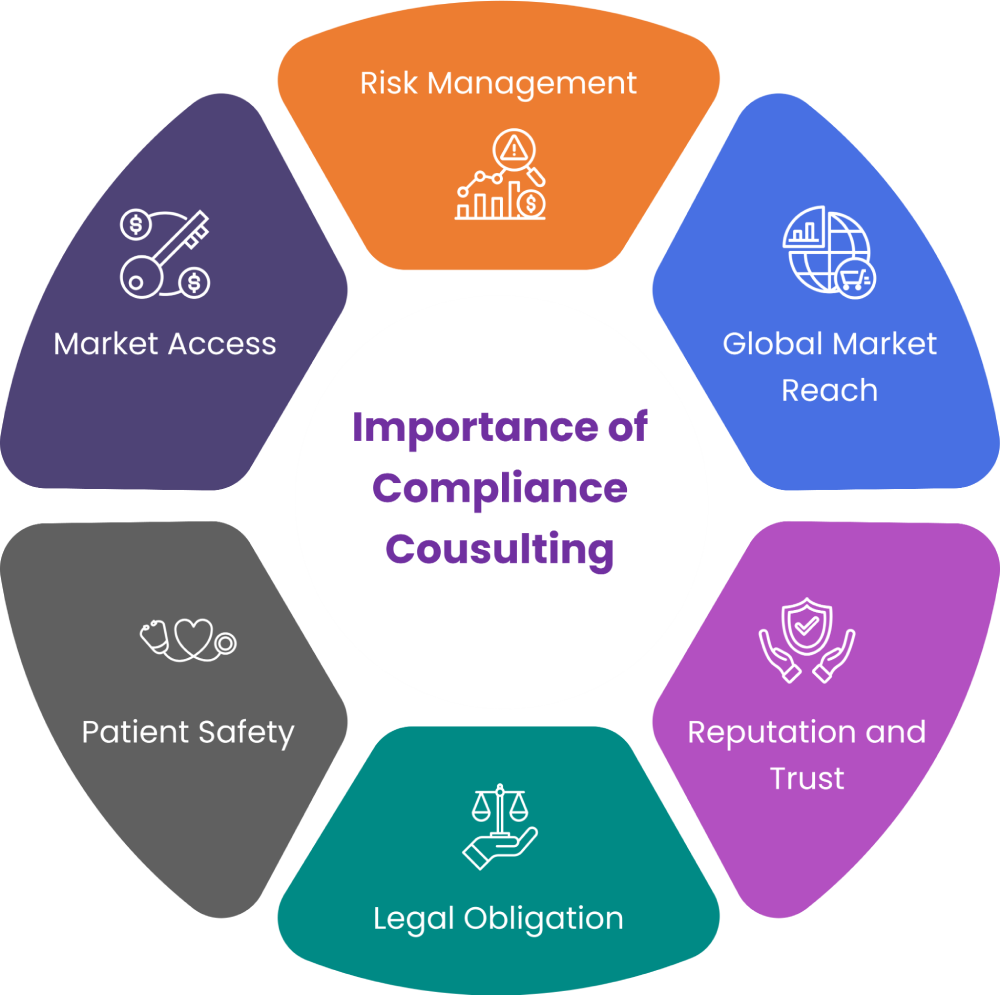
Figure 1: Importance of Compliance Consulting
Market Access:
Adhering to regulations such as the MDR or IVDR is essential for legally marketing medical devices within the European Union.
Non-compliance can lead to products being barred from the market, resulting in significant financial losses and operational disruptions.
Patient Safety:
Regulatory compliance ensures that medical devices meet stringent safety and performance standards.
This is vital for protecting patient health and minimizing risks associated with device malfunction or inadequacy.
Legal Obligation:
Compliance with frameworks like the MDR or IVDR is a legal requirement. Failure to comply can result in penalties, fines, and legal actions against the manufacturer.
Reputation and Trust:
Maintaining compliance enhances the reputation of medical device manufacturers.
It demonstrates a commitment to quality, safety, and ethical standards, fostering trust among healthcare professionals, patients, and regulatory bodies.
Global Market Reach:
Many countries outside the EU recognize or reference EU regulatory standards.
Compliance with EU regulations can facilitate easier access to other international markets, expanding the manufacturer’s global presence and customer base.
Risk Management:
Compliance involves rigorous assessment processes such as clinical evaluations and comprehensive technical documentation.
These processes help identify and mitigate potential risks associated with medical devices, thereby improving overall product quality and safety.
In essence, regulatory compliance is not merely a requirement but a cornerstone of responsible manufacturing in the medical device industry.
It ensures devices meet high standards of safety, efficacy, and quality, enabling manufacturers to access markets, protect patients, uphold their reputation, and mitigate risks effectively.
Compliance Consulting: Essential for Medical Device Registration
EU medical device consulting is indispensable for navigating the intricacies of EU medical device registration, ensuring manufacturers adhere meticulously to stringent regulatory requirements of the EU.
Here’s how compliance consultants contribute to the regulatory journey:
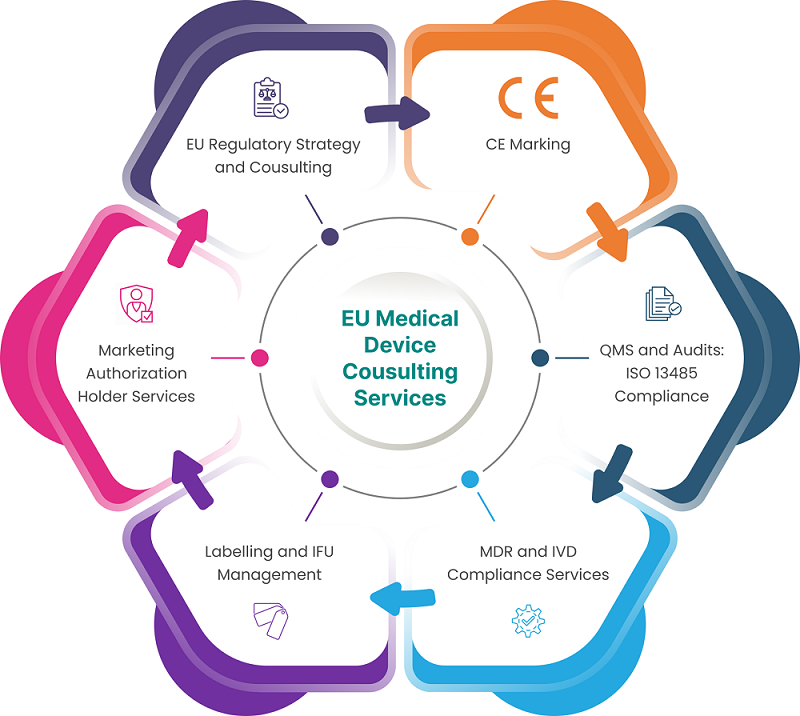
Figure 2: Compliance Consulting Services for Medical Device Registration in EU
- Regulatory Strategy Development: Consultants develop tailored regulatory strategies aligning with MDR requirements, optimising routes to market based on device classification and risk assessment.
- Device Classification: Experts ensure accurate classification of devices according to MDR risk categories, providing proper documentation and justification for classification decisions.
- Technical Documentation Preparation: Consultants assist in compiling comprehensive technical documentation demonstrating device safety, performance, and quality, meeting stringent MDR standards for notified body submission.
- Conformity Assessment Support: Services include guiding manufacturers through conformity assessment procedures tailored to device risk classes, ensuring compliance through audits and technical reviews.
- Unique Device Identification (UDI) System Implementation: Consultants facilitate UDI system implementation for traceability, assisting in assigning UDIs and managing submissions to EUDAMED for regulatory compliance.
- EUDAMED Registration Assistance: Support in registering devices and manufacturers in EUDAMED, ensuring accurate submission of device details and technical documentation for EU market access.
- Person Responsible for Regulatory Compliance (PRRC) Services: PRRC services appoint EU-based compliance officers, managing ongoing regulatory compliance and post-market surveillance activities for MDR adherence.
- Clinical Evaluation and Investigations: Consultants aid in designing and conducting clinical evaluations and investigations to validate device safety and performance under MDR requirements.
- Post-Market Surveillance and Vigilance: Services include developing post-market surveillance plans, reporting adverse events, and implementing corrective actions to maintain device safety and regulatory compliance.
By leveraging the expertise of compliance consultants, medical device manufacturers can streamline the regulatory process, mitigate the risks associated with non-compliance, and expedite market entry within the EU.
This approach not only ensures adherence to the highest standards of patient safety and product quality but also fosters trust and credibility in the global marketplace.
EU medical device consulting services play a crucial role in guiding manufacturers through these complexities, ensuring they meet regulatory standards efficiently and effectively.
Conclusion
EU Medical Device consulting is essential for navigating the complexities of EU medical device regulations.
It ensures that manufacturers meet rigorous standards for market approval, safeguarding patient safety and enhancing product quality.
By guiding through classification, QMS implementation, documentation preparation, and ongoing surveillance, consultants help streamline processes and mitigate risks.
This proactive approach not only facilitates market entry in the EU but also builds trust and credibility globally, ensuring devices meet the highest standards of safety and efficacy.
Summary
- Navigating European Union (EU) regulations like Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) is crucial for medical device manufacturers to ensure market approval and ongoing compliance.
- Consultants provide essential support in Quality Management Systems (QMS) implementation, technical documentation preparation, and engagement with Notified Bodies for certification.
- They play a pivotal role in proactive risk management and post-market surveillance, enhancing product safety and regulatory adherence.
- Adherence to EU regulations is pivotal for market access, avoiding legal penalties, and maintaining operational continuity.
- Compliance not only facilitates global market entry but also enhances reputation by demonstrating commitment to quality and safety standards.
References
- EU MDR and IVD Compliance, EU Medical Device Registration & Consulting Services | CliniExperts [Internet]. international.cliniexperts.com. [cited 2024 Jul 11]. Available from: https://international.cliniexperts.com/services/eu/mdr-and-ivd-compliance-regulations/
- Medicines and Medical Devices | Fact Sheets on the European Union | European Parliament [Internet]. www.europarl.europa.eu. 2024 [cited 2024 Jul 10]. Available from: https://www.europarl.europa.eu/factsheets/en/sheet/50/medicines-and-medical-devices#:~:text=Regulation%20%28EU%29%202017%2F745%20%28Medical%20Devices%20Regulation%29%20and%20Regulation
- EU Regulatory Strategy and Consulting – Medical Device & IVD | CliniExperts [Internet]. international.cliniexperts.com. [cited 2024 Jul 17]. Available from: https://international.cliniexperts.com/services/eu/eu-regulatory-strategy-and-consulting/
- Medical Device Registration in Europe – a Comprehensive Guide to EU MDR [Internet]. international.cliniexperts.com. 2024 [cited 2024 Jul 17]. Available from: https://international.cliniexperts.com/medical-device-registration-in-europe/
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree regulatory solutions to Medical Devices and In-Vitro Diagnostics.
CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




