Navigating the Complexities of EU Medical Device Consulting: How to Ensure Compliance
Summary:
The European Union (EU) medical device market is one of the largest and most stringent markets globally. To ensure patient safety and product efficacy, manufacturers must navigate complex regulations, particularly the EU Medical Devices Regulation (MDR) (2017/745) and the EU In Vitro Diagnostics Regulation (IVDR) (2017/746). This article delves into the challenges of medical device registration (EU) and provides a roadmap for achieving MDR compliance.

Understanding the Regulatory Landscape
The EU regulatory landscape for medical devices is dynamic and Complexities of EU medical device consulting. Consultants must stay current with the latest regulations, including amendments and interpretations. Key aspects to grasp include:
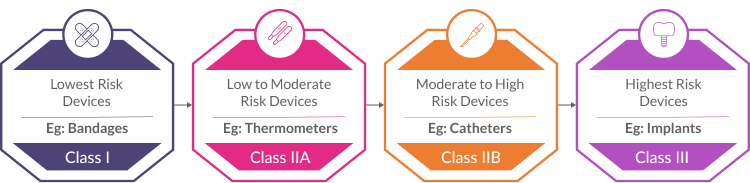
- Classification: Accurately classifying devices based on their intended purpose and risk level is crucial, as it determines the specific compliance requirements. To understand the risks and benefits of your device, the first step is to categorize it. According to the MDR, there are four categories of risk:
- Class I (Lowest Risk): Simple devices like bandages or tongue depressors.
- Class IIa (Low to Moderate Risk): Devices like thermometers or surgical drapes.
- Class IIb (Moderate to High Risk): Devices like catheters or infusion pumps.
- Class III (Highest Risk): High-risk devices like implants or life-sustaining equipment
- Clinical Evaluation: Robust clinical evidence demonstrating safety and effectiveness is essential for market access, and the required level of evidence varies based on the device class.
- Risk Management: Implementing a comprehensive risk management system to identify, assess, control, and monitor potential risks throughout the device lifecycle is mandatory.
- Technical Documentation: Maintaining thorough technical documentation that details design, development, manufacturing, and risk management processes is critical for demonstrating compliance.
- Post-Market Surveillance: Establishing a robust post-market surveillance system to continuously monitor device performance and identifying potential safety issues is crucial.
Challenges of EU Medical Device Consulting
- Complexity of Regulations: The sheer volume and intricate nature of the regulations can be overwhelming, even for experienced consultants.
- Continuous Updates: Keeping pace with frequent regulatory updates and changes requires ongoing learning and adaptation.
- Interpretation and Application: Accurately interpreting and applying regulations to specific devices can be challenging due to ambiguity in certain areas.
- Resource Constraints: Smaller companies might lack the in-house expertise and resources needed to navigate complex compliance processes.
Ensuring Compliance: A Roadmap for Success
- Engage Early: Partnering with a qualified consultant early in the development process allows for proactive compliance planning and avoids costly delays.
- Select the Right Consultant: Choose a consultant with proven expertise in EU medical device regulations and a strong understanding of your specific device type.
- Develop a Comprehensive Compliance Strategy: Collaborate with the consultant to develop a tailored compliance strategy that addresses all relevant regulatory requirements.
- Maintain Effective Communication: Ensure clear and consistent communication between the consultant, internal teams, and notified bodies throughout the process.
- Document Everything: Meticulously document all processes, decisions, and justifications throughout the development and compliance journey.
- Embrace Continuous Improvement: View compliance as an ongoing process and constantly seek opportunities to improve your quality management system and compliance posture.
Benefits of Utilizing EU Medical Device Consulting
- Reduced Risk of Non-Compliance: Consultants can help identify and mitigate potential compliance risks, saving time and money in the long run.
- Faster Market Access: By ensuring compliance from the outset, consultants can help expedite the market authorization process.
- Enhanced Product Safety: Consultants can guide companies in implementing robust risk management practices, ultimately leading to safer products for patients.
- Improved Quality Management Systems: Consultants can help establish and maintain effective quality management systems that foster continuous improvement and compliance.
Overview of the Medical Device Registration Process
| Stage | Description |
| Classification | Categorize devices based on risk (Class I-III) |
| Unique Device Identification (UDI) | Assign a unique identifier for traceability |
| EUDAMED Registration | Register the device and details in the EUDAMED database |
| Conformity Assessment | Follow procedures based on class (internal audit, notified body review, etc.) |
| Additional Considerations | Technical documentation, Person Responsible for Regulatory Compliance (PRRC), Post-Market Surveillance (reporting incidents, CAPA) |
Conclusion
Navigating the complexities of EU medical device consulting requires expertise, planning, and ongoing commitment. By partnering with qualified consultants and implementing a comprehensive compliance strategy, medical device manufacturers can ensure patient safety, gain market access, and achieve long-term success in the EU market.
References
- French-Mowat E. How are medical devices regulated in the European Union? J R Soc Med [Internet]. 2012;105 Suppl 1(1_suppl):S22-8. Available from: http://journals.sagepub.com/doi/10.1258/jrsm.2012.120036
- New Regulations [Internet]. Public Health. [cited 2024 Feb 29]. Available from: https://health.ec.europa.eu/medical-devices-sector/new-regulations_en
- Classification of Medical Devices and IVDs [Internet]. Eupati. eu. [cited 2024 Feb 29]. Available from: https://learning.eupati.eu/mod/page/view.php?id=935
- Conformity assessment [Internet]. Internal Market, Industry, Entrepreneurship and SMEs. [cited 2024 Mar 11]. Available from: https://single-market-economy.ec.europa.eu/single-market/goods/building-blocks/conformity-assessment_en
- Baines R et al. Navigating medical device certification: A qualitative exploration of barriers and enablers amongst innovators, notified bodies and other stakeholders. Ther Innov Regul Sci [Internet]. 2023;57(2):238–50. Available from: https://link.springer.com/10.1007/s43441-022-00463-4
- Canada GA. Six Steps to CE Marking [Internet]. GAC. 2019 [cited 2024 Mar 15]. Available from: https://www.tradecommissioner.gc.ca/guides/133383.aspx?lang=eng
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree Global Regulatory Solutions related to Pharma, Medical Devices and In-Vitro Diagnostics.
CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




