Common Challenges in Medical Devices QMS Audits and How to Overcome Them
Summary:
- QMS is vital for medical device compliance.
- ISO 13485 Auditing Services in the EU ensure quality management.
- Common challenges: internal audit gaps, leadership issues, lack of employee commitment, documentation challenges, cost and time constraints.
- Strategies: define clear objectives, align with strategic goals, integrate into planning, ensure ISO compliance, hire consultants, allocate resources, conduct a thorough audit

Introduction
A Quality Management System (QMS) is a structured framework that records processes, protocols, and duties to attain quality goals and directives. It aids in arranging and guiding an organisation’s operations to fulfil customer and regulatory standards while enhancing efficiency and efficacy over time.
This post about Challenges in Medical Devices QMS Audits. When it comes to medical devices, EU Quality Management services are involved in establishing systems and procedures to ensure the devices are safe, effective, and compliant with applicable regulations.
Medical device manufacturers aiming to market their products in Europe must adhere to ISO 13485. ISO 13485 Auditing Services in EU is an internationally acknowledged standard for quality management specific to the medical device sector. This standard is built upon ISO 9001 principles.
Significance of ISO 13485 Auditing Services in EU
Adhering to EU Quality Management Services is of paramount importance for medical device designers, manufacturers, and distributors.
ISO 13485 establishes criteria for quality management systems, ensuring these organisations demonstrate their ability to consistently deliver medical devices and associated services that meet customer requirements and regulatory mandates.
These organisations may be involved in various lifecycle stages, including design, storage, production, distribution, installation, servicing of medical devices, and related activities like technical support. Furthermore, ISO 13485 can benefit suppliers or external parties providing products or services related to the organisation’s offerings or quality management system.
Unless explicitly stated otherwise, the conditions of ISO 13485 Auditing Services in EU are relevant to organisations of all sizes and types. Whenever requirements pertain to medical devices, they also extend to associated services provided by the organisation.
Common Challenges in QMS Audits
While implementing EU Quality Management Services offers numerous advantages, it’s essential to plan the process meticulously. Here are some common quality management challenges that organisations often face:
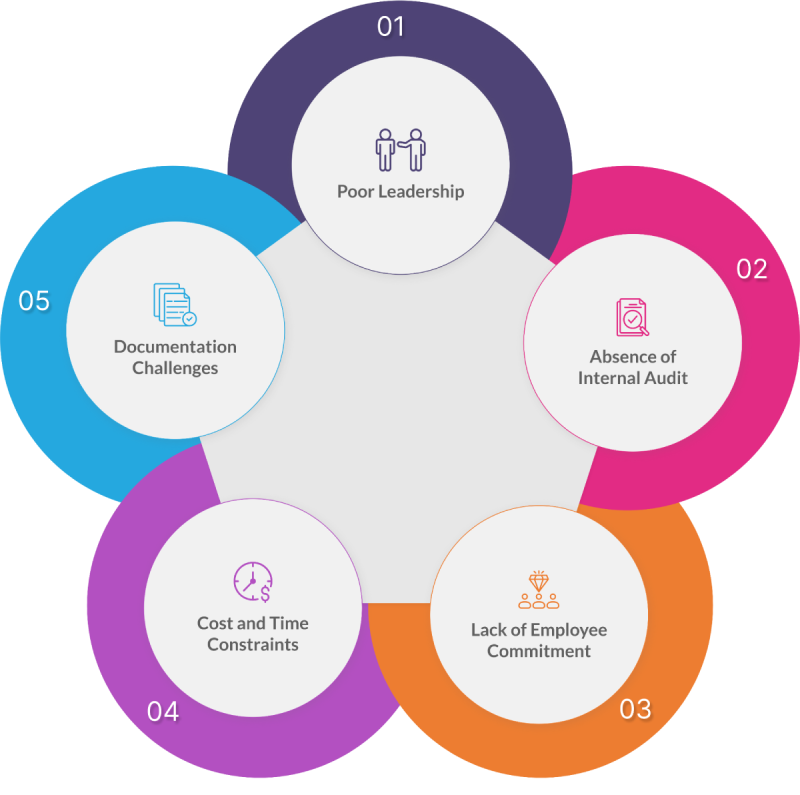
Poor leadership
Effective leadership is crucial for driving organisations towards quality improvement and excellence. Poor leadership can impede QMS implementation by creating confusion, undermining commitment, fuelling resistance to change,limiting resources, , and fostering a lack of accountability.
Absence of internal audit
Organisations are required to conduct internal audits at planned intervals to ensure compliance with standards and effective implementation of the quality management system.
But due to reasons like the perception of auditing as an additional workload, the tediousness of the process, lack of cooperation from auditees, and the absence of incentives can lead to failure to conduct internal audits resulting in a lack of insight into systemic issues with their products.
Lack of employee commitment
Lack of employee commitment can lead to resistance towards adopting QMS procedures and practices, resulting in non-compliance and inefficiencies.
Reduced motivation among employees may hinder the effective execution of quality-related tasks, such as adhering to documented procedures and participating in improvement initiatives.
Cost and time constraints
Cost and time constraints present significant hurdles for organisations when implementing a comprehensive QMS such as ISO 13485.
The process of adopting and adhering to the standards set by ISO 13485 can be time-consuming, involving thorough assessments, procedural adjustments and training sessions.
Documentation challenges
Limited knowledge and expertise pose challenges for organisations regarding ISO 13485 Auditing Services in EU. Additionally, extensive documentation mandated by ISO 13485 can be challenging for organisations with limited resources to manage effectively.
Strategies for Addressing Challenges in EU Quality Management Services
Numerous errors commonly encountered during the implementation of ISO 13485 processes are consistent throughout the industry and can be readily prevented. Here are a few strategies that can help:
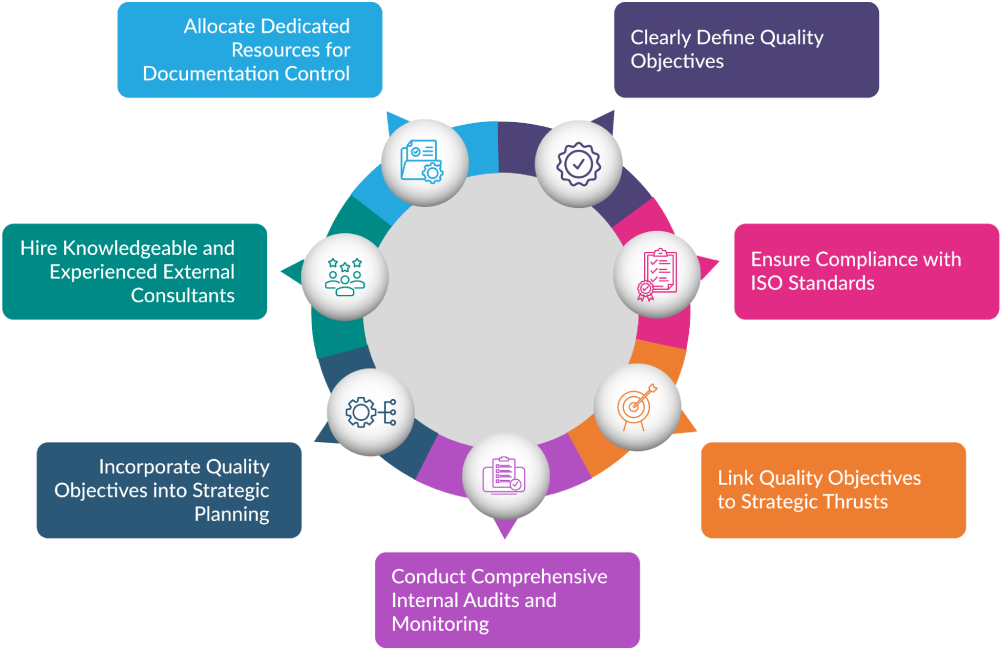
Clearly Define Quality Objectives
Top management should ensure that quality objectives are clearly defined and communicated throughout the organisation. These objectives should be measurable and consistent with the quality policy.
Ensure Compliance with ISO Standards
Organisations should incorporate their strategic initiatives and quality objectives into their quality manual, aligning them with ISO standards. This alignment ensures that the organization’s strategic plan adheres to both internal and external audit requirements.
Link Quality Objectives to Strategic Thrusts
Organisations can align their quality objectives with the goals set forth in their strategic plans. This alignment ensures that quality initiatives support and enhance broader organisational objectives.
Conduct comprehensive internal audits and monitoring
Conduct detailed internal audits and ongoing monitoring to ensure compliance with ISO 13485 Auditing Services in EU requirements, assess the effectiveness of the QMS, including risk management practices, and identify areas for corrective and preventive actions.
Incorporate Quality Objectives into Strategic Planning
Integrate quality objectives into the organisation’s strategic planning process. This integration helps prioritise quality initiatives and ensures they receive sufficient resources and attention.
Hire knowledgeable and experienced external consultants
To assist the organisation in understanding and implementing ISO 13485 requirements and to provide training to the management and employees on the standard.
Allocate dedicated resources for documentation control
Assign a person to manage document distribution, updates, and record retention and implement standardised formats and processes for documentation.
CliniExperts, a renowned regulatory consulting firm, provides comprehensive services designed to aid medical device manufacturers in navigating the implementation process of EU Quality Management Services.
With a skilled team of regulatory specialists and a deep understanding of industry standards, CliniExperts offers invaluable assistance at every step of the QMS implementation journey.
Additionally, CliniExperts’ guidance ensures adherence to regulatory guidelines, streamlines documentation processes, and enhances overall compliance with stringent quality standards, ultimately facilitating a successful and timely market entry for medical devices with ISO 13485 Auditing Services in EU.
Conclusion
In conclusion, implementing EU Quality Management Services presents both challenges and opportunities for medical device manufacturers.
By addressing common challenges in ISO 13485 Auditing Services in the EU such as internal audit gaps, leadership issues, and documentation challenges with strategic approaches and external expertise from firms like CliniExperts, organisations can enhance compliance, efficiency, and ultimately, market success.
References
- Quality management system. [Internet]. [cited 2024 May 14] Available from: https://asq.org/quality-resources/quality-management-system
- Quality management and medical devices. [Internet]. [cited 2024 May 14] Available from: https://www.tga.gov.au/how-we-regulate/manufacturing/manufacture-medical-device/quality-management-and-medical-devices
- Quality management. [Internet]. [cited 2024 May 14] Available from: https://ec.europa.eu/eurostat/web/quality/quality-monitoring/quality-management
- Medical Devices. [Internet]. [cited 2024 May 14] Available from: https://www.iso.org/iso-13485-medical-devices.html
- ISO 13485:2016. [Internet]. [cited 2024 May 14] Available from: https://www.iso.org/standard/59752.html
- Sanuri Mohd Mokhtar, S., Adiana Hiau Abdullah, N., Kardi, N., & Idzwan Yacob, M. (2013). Sustaining a quality management system: process, issues and challenges. Business Strategy Series, 14(4), 123–130. doi:10.1108/bss-12-2011-0032. Available from: https://sci-hub.se/https://doi.org/10.1108/BSS-12-2011-0032
- Hamimi Abdul Razak, I., Kamaruddin, S., Abdul Azid, I., & Putra Almanar, I. (2009). ISO 13485:2003. The TQM Journal, 21(1), 6–19. doi:10.1108/17542730910924718 Available from:https://sci-hub.se/https://doi.org/10.1108/17542730910924718
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree Global Regulatory Solutions related to Pharma, Medical Devices and In-Vitro Diagnostics.
CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




