Best Practices for Continuous Improvement in Medical Device Quality Management Systems
Summary:
- QMS documentation is vital for quality objectives and regulatory compliance in medical devices.
- Continuous improvement, with EU quality management services and ISO 13485 audits, enhances quality and ensures compliance.
- Optimal continuous improvement includes cultural and process balance, aligned leadership, data-driven decisions, and flexibility.
- Combining TQM, Six Sigma, Lean Thinking, or Theory of Constraints maximises improvement effectiveness.
- Fostering a continuous improvement culture demands leadership commitment, employee empowerment, and training.

Introduction
Quality management systems (QMS) are the backbone of ensuring quality and compliance in the medical device industry. This post is related to best practices for continuous improvement in medical device Quality Management Systems.
A QMS outlines the processes, procedures, and responsibilities for achieving quality objectives across all stages of a medical device’s lifecycle – from planning and design to manufacturing and commercialization.
Integrating with other business operations like procurement and resource management, a robust QMS promotes best practices supported by EU quality management services.
A QMS assists the manufacturer in providing sufficient employee training, ensuring compliance with customer requirements, adhering to legal and regulatory mandates, and monitoring key performance indicators for business execution, with assistance from ISO 13485 auditing services in the EU.
If any inefficiencies emerge in these areas, corrective measures are taken through established processes, followed by effectiveness assessments to confirm resolution. This ensures that any issues are promptly dealt with.
Significance of Continuous Improvement in EU Quality Management Services
The evolution of quality standards, such as ISO 9001, has increasingly emphasized customer satisfaction and continuous improvement. In more recent iterations like ISO 9001:2000, the focus has shifted from quality assurance to enhancing customer satisfaction.
In the context of medical device QMS, continuous improvement enables manufacturers to:
- Innovation and competitiveness: Continuously improving processes by adhering to ISO 13485 auditing services in EU can lead to cost savings, increased efficiency, and the development of innovative solutions, giving manufacturers a competitive edge.
- Regulatory adherence: Regulatory bodies, such as the FDA and EU MDR, emphasize the importance of continuous improvement in maintaining effective QMS.
- Enhance product quality and safety: By continuously refining processes and addressing non-conformances, manufacturers can improve product quality, reduce risks, and ensure patient safety.
Optimal Approaches for Continuous Improvement in QMS
Continuous improvement is essential for maintaining the effectiveness and relevance of QMS in today’s ever changing business environment.
By continuously refining the processes and practices of EU quality management services, organisations can increase efficiency, ensure customer satisfaction and enhance product quality.
Optimal approaches for continuous improvement in QMS encompass a range of strategies aimed at identifying areas for enhancement and implementing targeted solutions that include:
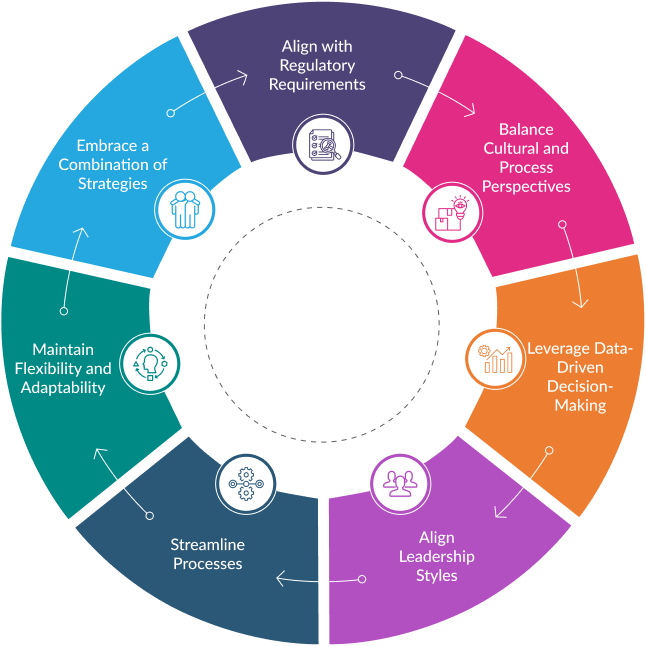
Align with Regulatory Requirements
Continuous improvement initiatives with evolving regulatory requirements, guidance documents, and industry best practices to maintain compliance and incorporate necessary changes into the QMS.
Balancing Cultural and Process Perspectives
Culture-focused approaches, such as Total Quality Management (TQM) and Lean Thinking, promote employee empowerment and shared knowledge, fostering a collective dedication to quality and betterment of QMS.
Meanwhile, process-focused methods like Six Sigma and Theory of Constraints ensure the establishment of robust systems and centralised expertise to optimise processes.
Leveraging Data-Driven Decision-Making
Effective continuous improvement hinges on data-driven decision-making and evidence-based problem-solving. Establishing robust data collection, analysis, and feedback mechanisms is crucial for identifying improvement opportunities in a QMS.
Aligning Leadership Styles
Effective leadership is essential for the successful implementation of proper QMS strategies. Leadership styles should correspond with the chosen strategy’s emphasis, whether cultural or process-oriented.
Streamline Processes
Continuously evaluate and optimise processes to improve efficiency, reduce waste, and enhance product quality by employing techniques like value process mapping, lean methodologies and stream mapping.
Maintaining Flexibility and Adaptability
The medical device industry is always evolving, characterised by changing regulations, technological advancements, and shifting market demands.
Continuous improvement approaches must be flexible and adaptable, enabling organisations to respond swiftly to changes and emerging challenges, while regular reviews and adjustments to improvement strategies are necessary to ensure their ongoing relevance and effectiveness.
Embracing a Combination of Strategies
To maximize effectiveness, it’s advisable to combine complementary continuous improvement strategies like TQM, Six Sigma, Lean Thinking, or Theory of Constraints.
Popular combinations include Lean Thinking paired with Six Sigma, Six Sigma coupled with Theory of Constraints, and TQM integrated with Theory of Constraints.
Nurturing a Culture of Continuous Improvement
Cultivating an organizational culture that values quality and champions change is essential for driving effective continuous improvement within a medical device QMS.
This mindset should be the core value of the organisation, with leadership demonstrating a commitment to continuous improvement and empowering employees to contribute ideas and solutions.
Regular training programmers and ongoing education initiatives play a vital role in ensuring employees understand the importance of continuous improvement, enhance their problem-solving abilities, and stay informed about industry advancements, all supported by ISO 13485 Auditing Services in EU.
Furthermore, acknowledging and rewarding successful improvement initiatives can reinforce positive behaviors and inspire enthusiastic participation in continuous improvement endeavors across the organization.
CliniExperts, a distinguished regulatory consulting firm, offers comprehensive services aimed at guiding medical device manufacturers through the integration of EU quality management services, facilitating adherence to best practices.
With a team of adept regulatory specialists and an in-depth understanding of industry standards, CliniExperts provides invaluable assistance at every stage of the QMS implementation journey.
Moreover, CliniExperts guidance ensures compliance with regulatory guidelines, streamlines documentation processes, and enhances overall adherence to stringent quality standards, thereby enabling manufacturers to uphold best practices.
Conclusion
In the fast-paced and strictly regulated medical device industry, continuous improvement is essential for maintaining an effective quality management system.
Embracing best practices for ongoing enhancement allows manufacturers to optimise processes, improve product quality and safety, and ensure compliance with regulations.
Through EU quality management services, medical device manufacturers are well-equipped to navigate complex regulatory requirements and elevate their quality management systems to meet international standards.
ISO 13485 auditing services in the EU allows the medical device companies to thoroughly evaluate their processes to ensure compliance with international quality standards.
By partnering with reputable auditing and management services like CliniExpert, manufacturers can position themselves as industry leaders, delivering medical devices that are safe, effective, and meet the highest standards of quality and regulatory compliance.
Reference
- Quality management system. [Internet]. [cited 2024 May 15] Available from: https://asq.org/quality-resources/quality-management-system
- Kristy Fearis, Aidan Petrie, Best practices in early phase medical device development: Engineering, prototyping, and the beginnings of a quality management system, Surgery, Volume 161, Issue 3,2017, Pages 571-575, ISSN 0039-6060, https://doi.org/10.1016/j.surg.2016.08.052. Available from: https://sci-hub.se/https://doi.org/10.1016/j.surg.2016.08.052‘
- Quality management and medical devices. [Internet]. [cited 2024 May 15] Available from: https://www.tga.gov.au/how-we-regulate/manufacturing/manufacture-medical-device/quality-management-and-medical-devices
- Brown, Alan & Eatock, Julie & Dixon, Dorian & Meenan, Brian & Anderson, John. (2008). Quality and continuous improvement in medical device manufacturing. The TQM Journal. 20. 541-555. 10.1108/17542730810909329. Available from: https://www.researchgate.net/publication/235323022_Quality_and_continuous_improvement_in_medical_device_manufacturing
- Hamimi Abdul Razak, I., Kamaruddin, S., Abdul Azid, I., & Putra Almanar, I. (2009). ISO 13485:2003. The TQM Journal, 21(1), 6–19. doi:10.1108/17542730910924718 Available from:https://sci-hub.se/https://doi.org/10.1108/17542730910924718
- Sanuri Mohd Mokhtar, S., Adiana Hiau Abdullah, N., Kardi, N., & Idzwan Yacob, M. (2013). Sustaining a quality management system: process, issues and challenges. Business Strategy Series, 14(4), 123–130. doi:10.1108/bss-12-2011-0032. Available from: https://sci-hub.se/https://doi.org/10.1108/BSS-12-2011-0032
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree regulatory solutions to Medical Devices and In-Vitro Diagnostics.
CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




