How to register your medical devices and IVDs in the UK
Summary:
- A manufacturer must register their medical devices and IVDs with the MHRA before putting them on the market in Great Britain.
- Registration of medical devices is governed by the Medical Devices Regulation 2002.
- All medical devices and IVDs must comply with the MDR 2002.
- A UK-based manufacturer or a single UK Responsible Person may register a medical device and IVD when intended to place on the Great Britain market.

Any manufacturer who intends to market their medical devices, including in-vitro diagnostics in the UK, must register medical devices with the Medicines and Healthcare Products Regulatory Agency (MHRA) before placing them into the markets in Great Britain (including England, Wales and Scotland). Manufacturers must ensure the devices have CE marks or UKCA markings before registration. A non-UK manufacturer can opt for a UK Responsible Person (UKRP) to act on their behalf to register devices.
This article overview the MHRA registration process, duration and expense needed for medical device registration in the UK.
Medical Devices Regulations, 2002
Medical Devices Regulations, 2002 or UK MDR, was enforced in the year 2002 and has undergone several amendments since then. UK MDR, 2002 has implemented directives for active implantable medical devices and in-vitro diagnostics. The UK MDR is active in the entire Great Britain region i.e., England, Wales and Scotland.
Who requires medical devices and IVD registration in United Kingdom?
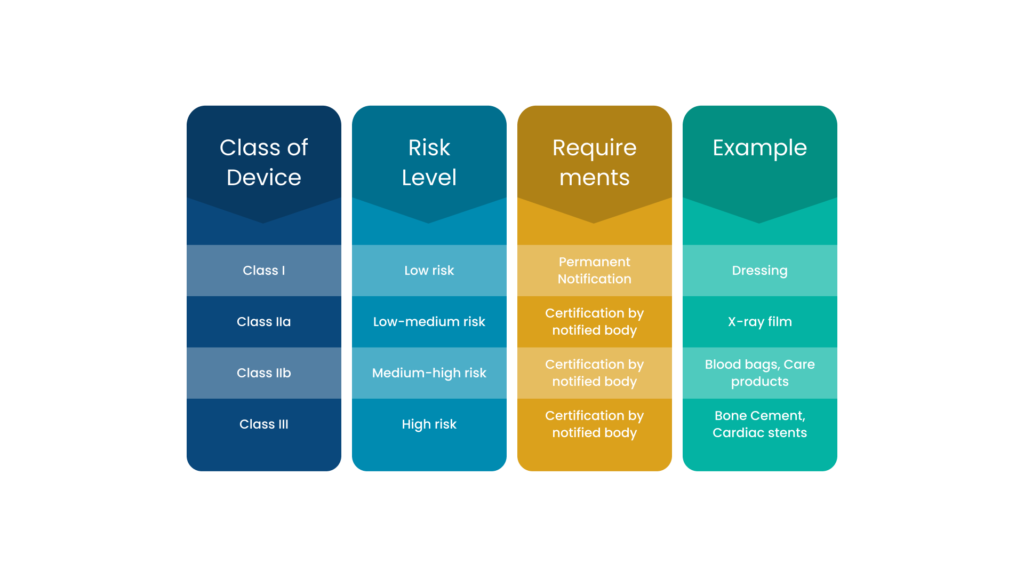
The MHRA classifies medical devices and IVDs into different categories, namely-
- Class I, IIa, IIb or III new medical devices and IVDs that are manufactured
- Class I, IIa, IIb or III devices refurbished or relabelled with a new name
- IVDs undergoing performance evaluation
- Any custom-made devices
- Any system or procedure pack comprising at least one medical device
Any manufacturer based in the UK who intends to place their products on the Great Britain market needs to apply for medical devices and IVD registration.
If a manufacturer is based outside of the UK, they can appoint a single UK Responsible Person to act on their behalf to file for the registration application for all of their medical devices and IVDs with the MHRA.
If an importer is based outside of the UK, similarly, they can appoint a UK Responsible Person and inform any relevant manufacturer of their intentions to import the devices into Great Britain. In such cases, the manufacturer or the Responsible Person must have all the importer’s details which also includes their place of business in Great Britain.
Distributors and suppliers need not register with the MHRA.
All medical devices, custom-made devices and systems or procedure packs, and IVDs, must be registered with the MHRA before they can be sold in the Great Britain market.
Essential Information for registering medical devices and IVDs registration with the MHRA
The registration process with the MHRA is relatively straightforward. This registration is more of a notification process and is not intended to validate any kind of device safety or efficacy.
According to the MHRA and UK MDR, 2002 regulation, a manufacturer is required to provide the following information while registering for medical devices and IVDs in Great Britain.
Manufacturer Details-
- An administrative contact (upto max 15 people)
- Company type (sole proprietary, etc)
- Name and address of a legal entity as it appears on the device labelling/packaging
- A letter of designation for UK Responsible Persons. It should be a legal contract specifying the mandatory tasks and contracting to act on behalf of a manufacturer.
Device details-
- Applicable legislation
- Device class
- Basic UDI-DI
- Medical device name
- Model or version detail
- UDI-DI
- Catalogue/ reference number
- UK Approved or EU Notified Body
- Global Medical Devices Nomenclature (GMDN) Code and Term describing the medical devices
- Attributes like contains latex, sterility, MRI compatibility
The application process for Medical Devices and IVDs registration
- The registration process is done through the Device Online Registration System (DORS).
- The manufacturer needs to create an account on the MHRA DORS portal before registering the medical devices.
- Upon registering, an email will be received to confirm if the account request has been accepted or rejected.
- While applying, the manufacturer must ensure all information registered with the MHRA is accurate and updated.
- MHRA may also request additional documents to demonstrate the medical devices conform with the regulatory requirement before registration is confirmed.
- Manufacturers are allowed to make any claims such as the use of MHRA logos in marketing materials, device packaging, instructions for use, laboratory tickets, or any other relevant documents.
- Registering medical devices with the MHRA does not permit any forms of accreditation, certification, approval or endorsement.
Once the registration is done with the MHRA, the manufacturers are granted the license to sell/distribute their medical devices into the UK market. These will be registered in the MHRA’s public registration database.
Expenses for Medical devices and IVDs registration
The MHRA charges a fee of £240 for each registration application. Manufacturers can register up to 100 medical devices and IVD registrations under the £240 fee if all are registered at the same time.
In case the manufacturer needs to update the information in the current registration, they may be charged an additional fee. This update may include changes in the registration from a UK Authorised Representative to a UK Responsible Person.
Timeline for registration approval
Upon registration on the DORS portal, MHRA takes up to five business days to review the application. The application review timelines may vary or can be extended as per MHRA.
In the review process, MHRA can request additional documents or more information related to classification rationale, request a copy of the instructions for use or images of the device. This would also extend the review time.
Registration Renewal
The MHRA has implemented a renewal process for registration to ensure it is up to date as per regulatory norms. Manufacturers must note the first renewal date to update the registration is one year after the request was completed by MHRA. Later at least every two years update is necessary.
MHRA has automated email delivery reminders for three, two and one month before your renewal date arises. Manufacturers can review the registration applications within three months. Renewal registration currently charges no fee.
Reference:
Register medical devices to place on the market – GOV.UK [Internet]. [cited 2023 Jul 4]. Available from: https://www.gov.uk/guidance/register-medical-devices-to-place-on-the-market
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions. We offer 360 degree Global Regulatory Solutions related to Pharma, Medical Devices and In-Vitro Diagnostics.
CliniExperts
CliniExperts Services Pvt. Ltd.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.




